The average speed of a diatomic chlorine molecule at 25 ∘C is 323.9 m⋅s−1 . What is the average wavelength of a chlorine molecule at this temperature? Assume that the molecule acts as a single particle.
I am confused about how to approach this problem. Because it says diatomic for the mass do we double the mass of chlorine? Also I saw that we have to use Avogadro's number but how do we just know that?
Achieve Question 17
Moderators: Chem_Mod, Chem_Admin
-
Erika Patel 3I
- Posts: 106
- Joined: Fri Sep 29, 2023 11:03 am
Re: Achieve Question 17
Hi, since this is a diatomic atom, you would double the atomic mass. Then you would need to convert to kg/mol (dividing grams by 1000 to get to kilograms). However, this is for 1 mol of chlorine molecules and we are being asked to find the wavelength for a single molecule. Since the conversion is 6.022x10^23 molecules/mol (Avogadro's number) we would divide the mass in kg by Avogadro's number. Then, you would plug this mass along with the given speed into the equation 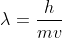 and solve for wavelength.
and solve for wavelength.
As to the second part of your question about just "knowing" to use Avogadro's number, I am not sure how to answer that but maybe someone else can.
I hope the steps above help you to solve!
As to the second part of your question about just "knowing" to use Avogadro's number, I am not sure how to answer that but maybe someone else can.
I hope the steps above help you to solve!
Re: Achieve Question 17
You would start by using the equation lambda=h/mv where we use Planck's constant, the mass of hydrogen by dividing its molar mass by Avogadro's numbers, and the given speed. With this equation, you can calculate the wavelength of a hydrogen molecule.
Return to “DeBroglie Equation”
Who is online
Users browsing this forum: No registered users and 1 guest

