Page 1 of 1
Question: How should I decide if I use DeBroglie Equation or the equation for Kinetic Energy?
Posted: Sun Oct 09, 2016 8:51 am
by E_Villavicencio 2N
Question #33 in the book says: The velocity of an electron that is emitted from a metallic surface by a photon is 3.6x10^3 km/s. What is the wavelenght of the ejected electron?
When I started solving the problem,
the first equation that came to my mind was the kinetic energy one (as the electron was ejected and this is moving), so I applied the formula
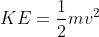
. However when comparing to the solutions, I noticed they used the DeBroglie Equation
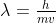
. The results were very different. How do I know when to use each of the equations?
Thanks for answering!
Re: Question: How should I decide if I use DeBroglie Equation or the equation for Kinetic Energy?
Posted: Sun Oct 09, 2016 10:11 am
by Shirley_Zhang 3O
Even thought both equations contain velocity, calculating Ek doesn't help you to solve for wavelength. At least with what we have learned so far, we do not know how to convert Ek to wavelength or frequency.
Re: Question: How should I decide if I use DeBroglie Equation or the equation for Kinetic Energy?
Posted: Sun Oct 09, 2016 2:04 pm
by E_Villavicencio 2N
I know, but what I did was to use the KE equation and then calculate the wavelenght using E=h(c/lamda)... Is that wrong?
Re: Question: How should I decide if I use DeBroglie Equation or the equation for Kinetic Energy?
Posted: Sun Oct 09, 2016 9:38 pm
by Chem_Mod
Hey Esmeralda! I can see why you are confused! The equation for energy that you used (E = hc/(lamda) is only used for light. For particles with mass, you would have to use the De Broglie equation.
Re: Question: How should I decide if I use DeBroglie Equation or the equation for Kinetic Energy?
Posted: Sun Oct 09, 2016 10:16 pm
by E_Villavicencio 2N
Ohhhhh, now everything is clear! Thank you very much!!!! :)
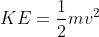 . However when comparing to the solutions, I noticed they used the DeBroglie Equation
. However when comparing to the solutions, I noticed they used the DeBroglie Equation 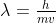 . The results were very different. How do I know when to use each of the equations?
. The results were very different. How do I know when to use each of the equations?