Page 1 of 1
De Broglie's Equation
Posted: Sun Oct 13, 2019 7:47 pm
by Sophia Dinh 1D
Why cant the De Broglie's equation be used for light?
Re: De Broglie's Equation
Posted: Sun Oct 13, 2019 7:49 pm
by Elena Bell 1C
De Broglie's equation requires an object to have a mass, therefore, the equation cannot be used for light because light does not have a mass.
Re: De Broglie's Equation
Posted: Sun Oct 13, 2019 7:52 pm
by Edmund Zhi 2B
The photons have no mass. Keep in mind that there is a similar situation in which we cannot apply equations for electromagnetic radiation to electrons. For example, we cannot use c = lambda nu for problems involving properties of electrons.
Re: De Broglie's Equation
Posted: Sun Oct 13, 2019 7:53 pm
by Tracy Tolentino_2E
De Broglie's equation is used for objects that has mass and behaves similarly to a wave. It also require momentum or velocity. Light theoretically doesn't have mass so the equation can't be used.
Re: De Broglie's Equation
Posted: Sun Oct 13, 2019 11:02 pm
by Ruth Glauber 1C
No, light cannot be used.
Re: De Broglie's Equation
Posted: Mon Oct 14, 2019 2:16 am
by Jillian C 4C
The equation requires an object that has mass, but light does not have that. Thus, light cannot be used in this equation.
Re: De Broglie's Equation
Posted: Mon Oct 14, 2019 1:30 pm
by Petrina Kan 2I
While all the previous replies have stated that light doesn't have mass and I agree, I just wanted to add that I remember Dr. Lavelle saying that the equation can only be used for particles with rest mass (mass at rest). It can be applied to everything else but light.
Re: De Broglie's Equation
Posted: Tue Oct 15, 2019 6:32 pm
by BCaballero_4F
Light does not have mass, which is a required variable in the De Broglie equation.
Re: De Broglie's Equation
Posted: Tue Oct 15, 2019 8:14 pm
by Leslie Almaraz 4G
De Broglie used light in order to derive the equation. The equation will work for any particle with momentum and it has a wavelength with its properties.
Re: De Broglie's Equation
Posted: Tue Oct 15, 2019 8:22 pm
by Zoya Mulji 1K
De Broglie suggested that the equation works for any particle with momentum, that has a rest mass, and has wavelike properties.
Re: De Broglie's Equation
Posted: Thu Oct 17, 2019 12:42 pm
by Debora Fernandez Clemente_ 4H
What is the difference between λ=h/p and c= λ*ν
Re: De Broglie's Equation
Posted: Thu Oct 17, 2019 12:56 pm
by 405268063
The first equation you've listed is the De Broglie equation and that's used for electrons or objects that have a mass. You cannot use light with the De Broglie equation. The second equation you CAN use for photons (light).
Re: De Broglie's Equation
Posted: Thu Oct 17, 2019 2:19 pm
by Megan Ngai- 3B
De Broglie's equation requires an object to have a mass
wavelength = h/mv
Re: De Broglie's Equation
Posted: Fri Oct 18, 2019 11:35 am
by 005321227
since wavelength=h/mv, the object must have a mass. While Light has a velocity, it does not have a mass to calculate
Re: De Broglie's Equation
Posted: Fri Oct 18, 2019 6:38 pm
by ValerieChavarin 4F
A photon does not have mass which is required for the de Broglie's equation where
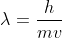
Re: De Broglie's Equation
Posted: Sat Oct 19, 2019 8:57 am
by Kaitlyn Jang 1F
You need to have mass to use the De Broglie Equation, however photons of light do not have mass.
Re: De Broglie's Equation
Posted: Sat Oct 19, 2019 2:45 pm
by HuyHa_2H
Photons don't have mass so the equation isn't applicable for it.
Re: De Broglie's Equation
Posted: Sun Oct 20, 2019 4:19 pm
by Emil Velasco 1H
Photons do not have mass, only momentum.