Given the energy, calculate the wavelength of y-rays
Moderators: Chem_Mod, Chem_Admin
-
Silvia Huang 3D
- Posts: 30
- Joined: Fri Sep 25, 2015 3:00 am
Given the energy, calculate the wavelength of y-rays
Hw questions 1.23 The y-ray photons emitted by the nuclear decay of a technetium-99 atom used in radiopharmaceuticals have an energy of 140.511 keV. Calculate the wavelength of these y-rays. I have no idea how to start this question... help please !! :)
-
Mariam Ghattas 2L
- Posts: 14
- Joined: Fri Sep 25, 2015 3:00 am
Re: Given the energy, calculate the wavelength of y-rays
In order for us to calculate the wavelength of the y rays given the the energy we will need to use a combined version of these two equations 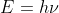
and c=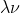
1st step is to look at the information given
we are given the energy but it is in KeV units we need to convert that to eV then to joules since energy is measured in joules for both equations needed for this problem
Kilo means 1000 of whatever unit is used. so in this case we multiply 140.511*10^3
conversion factor for eV to joules is 1eV=1.6022*10^{-19} J
(1.6022*10^{-19} J/eV)=2.2513*10^{-14}J)
since we now know the energy in joules we can combine the equations mentioned in the begining of the posts in order to get the wave length
equation. we will use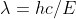
(2.99792*10^{8}m/s)}{2.2513*10^{-14}J}=8.8237*10^{-12}m or 8.8237pm)
and c=
1st step is to look at the information given
we are given the energy but it is in KeV units we need to convert that to eV then to joules since energy is measured in joules for both equations needed for this problem
Kilo means 1000 of whatever unit is used. so in this case we multiply 140.511*10^3
conversion factor for eV to joules is 1eV=1.6022*10^{-19} J
since we now know the energy in joules we can combine the equations mentioned in the begining of the posts in order to get the wave length
equation. we will use
-
Silvia Huang 3D
- Posts: 30
- Joined: Fri Sep 25, 2015 3:00 am
Re: Given the energy, calculate the wavelength of y-rays
Thank you very much!! I forgot that keV can be converted to joules... >_<
-
Mariam Ghattas 2L
- Posts: 14
- Joined: Fri Sep 25, 2015 3:00 am
Re: Given the energy, calculate the wavelength of y-rays
you are welcome , I hope my explanation helped :)
-
Nina Do 4L
- Posts: 61
- Joined: Fri Sep 28, 2018 12:27 am
Re: Given the energy, calculate the wavelength of y-rays
Hi,
First I converted the given keV to eV. I did that by multiplying 140.511 by 1000 and got 140511 eV. Then I used that and multiplied it by 1.602*10^-19 J because that is how many joules per eV. That gave me 2.251x10^-14 J for my energy. Then I set that equal to the (E=hc/lambda) equation to find wavelength. After some rearranging to get lambda alone, I'd then solve (hc/2.251x10^-19) which led to my answer of 883 nm. Hope that helped!
First I converted the given keV to eV. I did that by multiplying 140.511 by 1000 and got 140511 eV. Then I used that and multiplied it by 1.602*10^-19 J because that is how many joules per eV. That gave me 2.251x10^-14 J for my energy. Then I set that equal to the (E=hc/lambda) equation to find wavelength. After some rearranging to get lambda alone, I'd then solve (hc/2.251x10^-19) which led to my answer of 883 nm. Hope that helped!
-
Hadji Yono-Cruz 2L
- Posts: 64
- Joined: Fri Sep 28, 2018 12:26 am
-
Dakota Walker 1L
- Posts: 58
- Joined: Wed Sep 18, 2019 12:17 am
Re: Given the energy, calculate the wavelength of y-rays
How do you get the value for c in this equation?
-
Dakota Walker 1L
- Posts: 58
- Joined: Wed Sep 18, 2019 12:17 am
Re: Given the energy, calculate the wavelength of y-rays
also how do you convert the 10^-12 answer into nm
-
Ashley Osorio
- Posts: 54
- Joined: Thu Jul 11, 2019 12:17 am
Re: Given the energy, calculate the wavelength of y-rays
He got the value c from the speed of light.
-
Sean Cheah 1E
- Posts: 105
- Joined: Wed Sep 18, 2019 12:20 am
Re: Given the energy, calculate the wavelength of y-rays
Dakota Walker 1L wrote:also how do you convert the 10^-12 answer into nm
As stated above, the answer to your first question is that c in this case simply represents the speed of light, which is a constant that is approximately equal to
-
Camille 4I
- Posts: 57
- Joined: Sat Aug 24, 2019 12:18 am
Re: Given the energy, calculate the wavelength of y-rays
How do you change the answer value in meters to pm? The answer in the back of the book is in pm.
-
Camille 4I
- Posts: 57
- Joined: Sat Aug 24, 2019 12:18 am
Re: Given the energy, calculate the wavelength of y-rays
Also,
does the fact that the radiation consists of y-ray photons matter in this situation?
does the fact that the radiation consists of y-ray photons matter in this situation?
Re: Given the energy, calculate the wavelength of y-rays
Can someone please explain what keV means. Thank you.
Return to “DeBroglie Equation”
Who is online
Users browsing this forum: No registered users and 10 guests

