Electron Affinity Sapling
Moderators: Chem_Mod, Chem_Admin
-
DominicMalilay 1F
- Posts: 114
- Joined: Wed Sep 30, 2020 9:36 pm
Electron Affinity Sapling
For the electron affinity of thulium given 1064 nm and an energy of .137 ev, I'm confused how to find the electron affinity in ev/atom. I know it has something to do with debroglies equation, but I cant find the right units to solve it. Thanks in advance!
-
Edward Tang 1k
- Posts: 117
- Joined: Wed Sep 30, 2020 10:00 pm
- Been upvoted: 1 time
Re: Electron Affinity Sapling
Actually I dont think you need the wavelength equation. Just do e=(c/wavelength)*plancks to figure out the energy of the photon, convert that to eV by dividing 1.602e-19 and you're good
-
Sharon Kim 2A
- Posts: 104
- Joined: Wed Sep 30, 2020 9:42 pm
Re: Electron Affinity Sapling
For this question, it gave the energy value of the ejected electron in eV. To get the unit into eV/atoms do we just multiply by 6.022E23? I don't know if I would do that though because it is over moles. Is doing that technically making the units over atoms?
-
Gicelle Rubin 1E
- Posts: 101
- Joined: Fri Oct 02, 2020 12:16 am
Re: Electron Affinity Sapling
Edward Tang 2E wrote:Actually I dont think you need the wavelength equation. Just do e=(c/wavelength)*plancks to figure out the energy of the photon, convert that to eV by dividing 1.602e-19 and you're good
Literally thank you so much for this. I've been struggling with a similar question the entire week.
-
Ven Chavez 2K
- Posts: 101
- Joined: Mon Feb 24, 2020 12:16 am
Re: Electron Affinity Sapling
The question provides the ejection energy in eV and provides the wavelength of the incident radiation. Electron affinity is calculated by finding the difference in energy between the incident photons and the ejected electrons. First, you find the energy of the incident radiation using a modified version of E=hv or 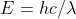 . This provides you the energy in Joules which you then convert to eV. Then you subtract the energy of the ejected electrons from the energy of the incident radiation. Part two of the question asks you to convert the electron affinity into kJ/mol. Remember that the equation E=hv gives the energy in joules of one photon. Therefore to convert to kJ/mol, you take the electron affinity from part one, convert it back into joules and then into kilojoules, and finally convert atoms to moles.
. This provides you the energy in Joules which you then convert to eV. Then you subtract the energy of the ejected electrons from the energy of the incident radiation. Part two of the question asks you to convert the electron affinity into kJ/mol. Remember that the equation E=hv gives the energy in joules of one photon. Therefore to convert to kJ/mol, you take the electron affinity from part one, convert it back into joules and then into kilojoules, and finally convert atoms to moles.
-
Jasmine Yi 1G
- Posts: 100
- Joined: Wed Sep 30, 2020 9:37 pm
Re: Electron Affinity Sapling
Edward Tang 2E wrote:Actually I dont think you need the wavelength equation. Just do e=(c/wavelength)*plancks to figure out the energy of the photon, convert that to eV by dividing 1.602e-19 and you're good
Just making sure, we have to do the conversion because e=(c/wavelength)*plancks gives us an answer in joules, right?
-
Edward Tang 1k
- Posts: 117
- Joined: Wed Sep 30, 2020 10:00 pm
- Been upvoted: 1 time
Re: Electron Affinity Sapling
Jasmine Yi 1H wrote:Edward Tang 2E wrote:Actually I dont think you need the wavelength equation. Just do e=(c/wavelength)*plancks to figure out the energy of the photon, convert that to eV by dividing 1.602e-19 and you're good
Just making sure, we have to do the conversion because e=(c/wavelength)*plancks gives us an answer in joules, right?
Yep you're absolutely right.
Return to “DeBroglie Equation”
Who is online
Users browsing this forum: No registered users and 3 guests

