Why do we use kgs in DeBrogile's equation?
Moderators: Chem_Mod, Chem_Admin
Why do we use kgs in DeBrogile's equation?
Hi! I just watched Dr. Lavelle's Friday Lecture on Debroglie's equation and did not understand why we use kgs for the mass in the equation...Isn't grams the SI unit?
Could someone tell me if I am confusing something here!
Nicolena
Could someone tell me if I am confusing something here!
Nicolena
-
Jessica Phung 1E
- Posts: 56
- Joined: Fri Sep 24, 2021 6:25 am
Re: Why do we use kgs in DeBrogile's equation?
HI! I think we use kg because in this equation, the constants are in terms of kgm/s.
-
Brandon Yu
- Posts: 121
- Joined: Fri Sep 24, 2021 6:07 am
Re: Why do we use kgs in DeBrogile's equation?
We use kg as opposed to g because kg is the standard unit for mass and you want to convert to SI units before doing calculations!
-
Tess_Fleser_1D
- Posts: 66
- Joined: Fri Sep 24, 2021 5:40 am
Re: Why do we use kgs in DeBrogile's equation?
Yup!
Mass is kind of like the exception to the rule of SI base units. Kg is technically the base unit, though there are some equations that use grams.
The easiest way to know you need to use kg in this situation is because it involves J, and J is kgm^2s^-2, so we need kg not g.
Mass is kind of like the exception to the rule of SI base units. Kg is technically the base unit, though there are some equations that use grams.
The easiest way to know you need to use kg in this situation is because it involves J, and J is kgm^2s^-2, so we need kg not g.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Why do we use kgs in DeBrogile's equation?
Hello,
We use the kilogram in the equations because (1) it is an SI Unit and (2) is allows the corresponding units to cancel.
(\frac{m^{2}}{s^{2}})) and
and (\frac{m}{s}))
(\frac{m^{2}}{s^{2}})(s)}{(kg)(\frac{m}{s})} = meters)
We use the kilogram in the equations because (1) it is an SI Unit and (2) is allows the corresponding units to cancel.
-
Preethika Praveen 2G
- Posts: 100
- Joined: Fri Sep 24, 2021 6:25 am
Re: Why do we use kgs in DeBrogile's equation?
Hi! In order to use the De Broglie equation (wavelength=h/p) you need to convert to kilograms in order to cancel out the Joules in Planck's constant (which is measured in kg m^2 s^-2 ).
-
Jonathan Shyu 3L
- Posts: 102
- Joined: Fri Sep 24, 2021 5:07 am
Re: Why do we use kgs in DeBrogile's equation?
We use Kgs to allow for calculations across formulas not only in chem but physics and any other subjects that apply to be applicable. Kgs are a part of the SI units, which are the standard units that everyone utilizes during calculations to allow for consistent and accurate outcomes.
-
masontsang
- Posts: 52
- Joined: Fri Sep 24, 2021 5:09 am
Re: Why do we use kgs in DeBrogile's equation?
Hi!
Kilograms (kg) are the SI unit for mass, so you should convert grams to kilograms before doing the calculations. Tess brought up a really good point too! Since one Joule = kgm^2/s^2, and Joules were the unit given in the problem, it would make the most sense to use kg in your calculations!
I hope this helps!
Kilograms (kg) are the SI unit for mass, so you should convert grams to kilograms before doing the calculations. Tess brought up a really good point too! Since one Joule = kgm^2/s^2, and Joules were the unit given in the problem, it would make the most sense to use kg in your calculations!
I hope this helps!
-
Matthew Li 1B
- Posts: 118
- Joined: Fri Sep 24, 2021 6:40 am
Re: Why do we use kgs in DeBrogile's equation?
we use kg in debrogiles equation because we use kg to calculate momentum
-
Megan Cai 1H
- Posts: 51
- Joined: Fri Sep 24, 2021 6:53 am
Re: Why do we use kgs in DeBrogile's equation?
Hi! We use kilograms so that the units can cancel with Joules (which has kilograms as a part of its units).
-
Kirsten Yu 2K
- Posts: 82
- Joined: Fri Sep 24, 2021 5:10 am
Re: Why do we use kgs in DeBrogile's equation?
The reason we use kg instead of g is because kg is the SI unit for mass.
-
Eric Sun 2G
- Posts: 102
- Joined: Fri Sep 24, 2021 5:08 am
Re: Why do we use kgs in DeBrogile's equation?
1 J = 1 kg*m^2/s^2. For this reason, we would try to convert our mass values to kg in order to cancel out the units.
-
Meia Schram 1J
- Posts: 52
- Joined: Fri Sep 24, 2021 6:03 am
- Been upvoted: 1 time
Re: Why do we use kgs in DeBrogile's equation?
Hi,
We use kg for mass because it will cancel out nicely with Jules which has kg as well. Hope this helps!
We use kg for mass because it will cancel out nicely with Jules which has kg as well. Hope this helps!
-
Peter Fernandez 2K
- Posts: 56
- Joined: Fri Sep 24, 2021 6:21 am
Re: Why do we use kgs in DeBrogile's equation?
Kg is used instead of g because it is the standard SI unit for mass!
-
Phillip Ma 1J
- Posts: 100
- Joined: Fri Sep 24, 2021 7:05 am
Re: Why do we use kgs in DeBrogile's equation?
Hi 205743684! The units for Planck's Constant is kg*m^2/s. Because kg are the units in the equation, you must have mass in kg.
-
Skylar Lo 2C
- Posts: 110
- Joined: Fri Sep 24, 2021 5:10 am
Re: Why do we use kgs in DeBrogile's equation?
We use kg in chemistry because it is the SI unit of mass.
-
Jennifer Fuentes 2K
- Posts: 99
- Joined: Fri Sep 24, 2021 6:17 am
Re: Why do we use kgs in DeBrogile's equation?
Joules is measured in kg m^2 s^-2. Therefore, in order for the De Broglie Equation to work you have to use a mass with kilograms in order to cancel out with the kg in Joules in Planck's Constant.
-
Ruben Adamov 1E
- Posts: 105
- Joined: Fri Sep 24, 2021 5:56 am
Re: Why do we use kgs in DeBrogile's equation?
Because 1 J = 1 kg*m^2/s^2, we convert our units to kg so we can cancel them out in the equation.
Hope this helps!
Hope this helps!
-
Janys Li - 1L
- Posts: 52
- Joined: Fri Sep 24, 2021 5:13 am
Re: Why do we use kgs in DeBrogile's equation?
Hi!
Kg is the SI unit for most calculations. When calculating molecular weights, you often use grams, but you would only do it if that is specified.
Kg is the SI unit for most calculations. When calculating molecular weights, you often use grams, but you would only do it if that is specified.
-
Shriya_Amara_1G
- Posts: 100
- Joined: Fri Sep 24, 2021 7:28 am
Re: Why do we use kgs in DeBrogile's equation?
De Broglie's equation is 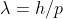
h stands for Planck's constant, which is in terms of Joules per Hertz
Joules per Hertz is technically in terms of (kg⋅m2⋅s−1)
So for the kg in Planck's constant to cancel out the mass in the momentum equation (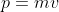 ), the mass has to be in kg.
), the mass has to be in kg.
Also, kg is technically the SI Unit, but grams is used in some equations too.
h stands for Planck's constant, which is in terms of Joules per Hertz
Joules per Hertz is technically in terms of (kg⋅m2⋅s−1)
So for the kg in Planck's constant to cancel out the mass in the momentum equation (
Also, kg is technically the SI Unit, but grams is used in some equations too.
-
Neelaj Das 3I
- Posts: 103
- Joined: Fri Sep 24, 2021 5:35 am
Re: Why do we use kgs in DeBrogile's equation?
If you notice, in De Broglie's wave equation Planck's constant (h) is in the denominator. The units for this constant are Joules times seconds, and joules involves kilograms so that's why the mass also has to be kilograms.
-
Sidharth Paparaju 3B
- Posts: 167
- Joined: Fri Sep 24, 2021 5:14 am
Re: Why do we use kgs in DeBrogile's equation?
We use kgs because the units for planck's constants include J, which is defined as kgm^2/s^2, so to cancel out the kg, we need mass in kg.
-
Aryan Gajjar 3D
- Posts: 114
- Joined: Fri Sep 24, 2021 6:05 am
- Been upvoted: 1 time
-
August Blum Dis 3D
- Posts: 54
- Joined: Fri Sep 24, 2021 5:30 am
Re: Why do we use kgs in DeBrogile's equation?
We use kg because it is the SI unit necessary to cancel out the kg in Joules.
-
Harsimer Bal 3K
- Posts: 106
- Joined: Fri Sep 24, 2021 5:16 am
- Been upvoted: 1 time
Re: Why do we use kgs in DeBrogile's equation?
I was also very confused on why we used kg instead of g until I found out that kg is the SI unit for mass!
-
Michelle Li 2B
- Posts: 109
- Joined: Fri Sep 24, 2021 5:11 am
- Been upvoted: 1 time
Re: Why do we use kgs in DeBrogile's equation?
Hi! We use kg because Joules are kgm^2/s^2, which are in Planck's constant which is in the numerator. Therefore, the mass is in the denominator so you want to use kg to cancel out the kg in the numerator.
-
Victoria Sigala 2A
- Posts: 50
- Joined: Fri Sep 24, 2021 5:50 am
Re: Why do we use kgs in DeBrogile's equation?
In De Broglie's equation we use kg for mass in order to be able to cancel the Jules from Planks constant as it is (kg)(m^2/s^2).
-
Celine Khuu 2F
- Posts: 100
- Joined: Fri Sep 24, 2021 6:31 am
Re: Why do we use kgs in DeBrogile's equation?
We use kilograms for mass because the SI units of a joule is kg*m^2*s^-2.
-
Tanvi Akula 2K
- Posts: 95
- Joined: Fri Sep 24, 2021 6:41 am
Re: Why do we use kgs in DeBrogile's equation?
The units for energy are joules, and joules are equal to kgm^2/s^2. So since DeBroglie's equation is derived from energy and because Planck's constant also uses joules, we use kgs in Debroglie's equation.
-
Emily Wu 2G
- Posts: 99
- Joined: Fri Sep 24, 2021 6:23 am
Re: Why do we use kgs in DeBrogile's equation?
We use Kg because of moles but you can always convert g to kg !
-
Santiago Chang 2K
- Posts: 54
- Joined: Fri Sep 24, 2021 6:55 am
Re: Why do we use kgs in DeBrogile's equation?
We use kg for the mass in De Broglie's equation because the equation helps to determine energy in Joules, which is kgm^2/s^2. The units need to match on both sides. Also, kg is the SI unit for mass. Hope this helps!
-
Gabby Burgess 2I
- Posts: 101
- Joined: Fri Sep 24, 2021 5:34 am
Re: Why do we use kgs in DeBrogile's equation?
Usually kg is used because it is the SI unit for chemistry!
Return to “DeBroglie Equation”
Who is online
Users browsing this forum: No registered users and 14 guests

