De Broglie Wavelength vs. Normal Wavelength
Moderators: Chem_Mod, Chem_Admin
-
carterwink2K
- Posts: 100
- Joined: Fri Sep 24, 2021 5:57 am
- Been upvoted: 1 time
De Broglie Wavelength vs. Normal Wavelength
Hey guys. I'm confused as to the difference between a De Broglie wavelength and a normal wavelength. Is there even a difference?
-
Jack Amos 2I
- Posts: 106
- Joined: Fri Sep 24, 2021 7:31 am
Re: De Broglie Wavelength vs. Normal Wavelength
Hi! I think I understand what you're trying to get at. Any particle has a De Broglie wavelength, from an electron to something like an elephant, they both have some wavelength. This wavelength (for any particle) is calculated by using De Broglie's equation: 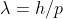 . Light, radiation, photon are oddities. Although they act particle-like, we don't calculate the wavelength of light (photons) with the De Broglie equation. This is because light does not have measurable mass and due to this, we must use either:
. Light, radiation, photon are oddities. Although they act particle-like, we don't calculate the wavelength of light (photons) with the De Broglie equation. This is because light does not have measurable mass and due to this, we must use either: /\lambda) or
or  . So, to answer your question, there is De Broglie wavelength and wavelength of light.
. So, to answer your question, there is De Broglie wavelength and wavelength of light.
-
Alex_Lee_1K
- Posts: 105
- Joined: Fri Sep 24, 2021 5:26 am
Re: De Broglie Wavelength vs. Normal Wavelength
The main difference between De Broglies wave length and "normal wavelength" is that when you use De Broglie's you are finding the wavelength of an electron, while what I assume is "normal wavelength" is Ephoton = (hc)/lambda is finding the wavelength of a photon.
-
Jane Wang 1E
- Posts: 115
- Joined: Fri Sep 24, 2021 6:36 am
Re: De Broglie Wavelength vs. Normal Wavelength
Hi! Objects with mass can have wavelike properties when they have motion, and the wavelength they have is called De Broglie wavelength, like they are not originally electromagnetic wave, but they are masses with wavelike properties, hence have wavelength. But normal wavelength describes the wavelength of electromagnetic wave like visible light etc.
Hope this helps!
Hope this helps!
-
Madison Yee 2B
- Posts: 101
- Joined: Fri Sep 24, 2021 5:33 am
Re: De Broglie Wavelength vs. Normal Wavelength
Hi there! The De Broglie wavelength is generally used to measure anything with a rest mass. In our case, we have been using it to measure the wavelength of electrons. We use the equation c = lambda * nu to measure the wavelength of light since light has a speed of 3.00 * 10^8. Furthermore, since photons don't have a rest mass (a mass when it is at rest) we can't use the de Broglie wavelength equation to determine the wavelength of a photon since we won't know the particle's mass. Hope this helps!
-
Prithvi Raj 3E
- Posts: 106
- Joined: Fri Sep 24, 2021 5:46 am
Re: De Broglie Wavelength vs. Normal Wavelength
For one, a photon does not have a de Broglie wavelength because it does not have a mass, giving it no momentum. Additionally, something could have a wavelength, but no wavelike properties. This is when your wavelength is lower than 10^-15m. If your wavelength is lower than this, you won't have any noticeable wave properties.
-
Jayden Tan 2L
- Posts: 52
- Joined: Fri Sep 24, 2021 7:32 am
Re: De Broglie Wavelength vs. Normal Wavelength
Hello!!
From my knowledge and the UA sessions, the De Broglie wavelength is used to find the wavelength of an electron, which has a mass!! (The equation really measures the wavelength of anything with a mass, but we need to ensure that it is even measurable!) Conversely, the normal wavelength is for photons, which carry no mass! Hope this helped :))
From my knowledge and the UA sessions, the De Broglie wavelength is used to find the wavelength of an electron, which has a mass!! (The equation really measures the wavelength of anything with a mass, but we need to ensure that it is even measurable!) Conversely, the normal wavelength is for photons, which carry no mass! Hope this helped :))
-
Ishpreet Kaur 3C
- Posts: 52
- Joined: Fri Sep 24, 2021 6:37 am
Re: De Broglie Wavelength vs. Normal Wavelength
The reason there's a distinction is likely just because of the sheer speed of a photon since once you get to that speed the physics gets wonky.
Return to “DeBroglie Equation”
Who is online
Users browsing this forum: No registered users and 4 guests

