Psi in the Equation
Moderators: Chem_Mod, Chem_Admin
-
Matlynn Giles 2E
- Posts: 104
- Joined: Wed Sep 30, 2020 10:10 pm
- Been upvoted: 1 time
-
Peter DePaul 1E
- Posts: 101
- Joined: Wed Sep 30, 2020 9:55 pm
- Been upvoted: 1 time
Re: Psi in the Equation
I believe for our purposes we don't have to use 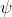 or
or 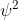 . If we do have to use one of the two though I'm gonna guess it would be
. If we do have to use one of the two though I'm gonna guess it would be 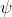 , because
, because 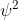 is for the probability density of finding an electron in that location so I don't see how that would be used in the equation.
is for the probability density of finding an electron in that location so I don't see how that would be used in the equation.
-
Megan Sparrow 1A
- Posts: 115
- Joined: Wed Sep 30, 2020 9:49 pm
- Been upvoted: 4 times
Re: Psi in the Equation
For the Heisenberg equation, we haven't used psi I believe. Psi is used in Schrodinger's equation and represents the wave function. Psi squared represents the probability density of an electron. That tells us where the electron is most likely to be found in the space around the nucleus. If you want to know more about this equation, I found the information here useful.
http://hyperphysics.phy-astr.gsu.edu/hb ... /schr.html
http://hyperphysics.phy-astr.gsu.edu/hb ... /schr.html
- Attachments
-
- download-1.png (2.13 KiB) Viewed 570 times
-
Sana Nagori 2H
- Posts: 149
- Joined: Wed Sep 30, 2020 9:43 pm
- Been upvoted: 1 time
Re: Psi in the Equation
I don't think we do use it in Heisenberg. I only remember seeing it for Schrodinger where psi is the wave function
-
allyssa bradley 1H
- Posts: 95
- Joined: Wed Sep 30, 2020 9:47 pm
Re: Psi in the Equation
I'm pretty sure Heisenberg relates to the particle property and psi relates to the wave property, so I don't think they would overlap.
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 2 guests

