Hi!!
I have a question regarding the Uncertainty principle. If given a problem where the momentum uncertainty, for example, is + or - 2, does that mean the uncertainty amount is 4? Or do you have to take into account the value of the momentum?
Thanks!
Uncertainty Principle [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Janice Xiao 1I
- Posts: 52
- Joined: Thu Jul 13, 2017 3:00 am
Re: Uncertainty Principle
The uncertainty amount is 4. You don't need to take into account the actual momentum value.
-
Brandon Fujii 1K
- Posts: 51
- Joined: Fri Sep 29, 2017 7:04 am
Re: Uncertainty Principle
Also if you use number 1.45 as a practice problem, the solutions manual is incorrect. The actual velocity given is 5.00 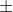 5.0m/s
5.0m/s
Thus,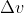 =10.0m/s NOT 5.0m/s as the solution manual incorrectly states. Therefore, your answer is
=10.0m/s NOT 5.0m/s as the solution manual incorrectly states. Therefore, your answer is 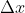 =6.7x10-37m
=6.7x10-37m
Thus,
-
Charles Ang 1E
- Posts: 50
- Joined: Thu Jul 13, 2017 3:00 am
Re: Uncertainty Principle [ENDORSED]
An easy way to remember this is to think of delta v as the change in v like in math. So, we arent concerned with the value of v, but rather the difference between the two limits to its domain.
-
nanditasundarapandian1D
- Posts: 21
- Joined: Sat Jul 22, 2017 3:01 am
-
Richard Braun 1I
- Posts: 65
- Joined: Fri Sep 29, 2017 7:05 am
Re: Uncertainty Principle
The uncertainty is 4. If the problem states +/- 2, that means 2 from 0 both ways, added together, gives you four.
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 2 guests

