h-bar [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Jade Corpus-Sapida 1G
- Posts: 35
- Joined: Fri Apr 06, 2018 11:02 am
h-bar
In the Heisenberg uncertainty equation, does h-bar basically mean plancks constant divided by 2pi? Will this number always be the same/constant or are there instances where its different?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
-
Jacob Samuels 1E
- Posts: 30
- Joined: Fri Apr 06, 2018 11:05 am
-
Caroline Crotty 1D
- Posts: 32
- Joined: Fri Apr 06, 2018 11:02 am
Re: h-bar
Since on Test #1 we are given many formulas we did not need for that test specifically, I think we can assume h bar will not be specifically given. The formula sheet does include the value of Planck's constant and pi so you can easily use that information and your knowledge of h bar to find the value needed.
-
EllenRenskoff-1C
- Posts: 32
- Joined: Fri Apr 06, 2018 11:04 am
Re: h-bar
If h-bar is h/ 2*pi, where does the h/ 4*pi come from in the Heisenberg Indeterminancy Function come from?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: h-bar
h-bar is used because it is a common value for use with the Schroedinger equation. 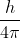 is a derived quantity in the Heisenberg equation, but it is not represented with a special character because it does not show up as commonly in quantum mechanical equations compared to h-bar.
is a derived quantity in the Heisenberg equation, but it is not represented with a special character because it does not show up as commonly in quantum mechanical equations compared to h-bar.
-
Emma Leshan 1B
- Posts: 30
- Joined: Fri Apr 06, 2018 11:03 am
Re: h-bar
The Uncertainty Equation stated in the textbook has 1/2 * h-bar, and since h-bar is h/2pi, we use h/4pi.
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 3 guests

