Uncertainty in Speed [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Veronica_Lubera_2A
- Posts: 106
- Joined: Sat Sep 14, 2019 12:16 am
- Been upvoted: 1 time
Uncertainty in Speed
The hydrogen atom has a radius of approximately 0.05 nm. Assume that we know the position of an electron to an accuracy of 1% of the hydrogen radius, calculate the uncertainty in the speed of the electron using the Heisenberg uncertainty principle.
So the correct answer is Delta V >= 10^8 m/s. How would you solve the question to receive this answer?
So the correct answer is Delta V >= 10^8 m/s. How would you solve the question to receive this answer?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Uncertainty in Speed [ENDORSED]
I will be covering this topic in class during Week 2.
Calculate 1% of the hydrogen radius (0.05 nm) in meters and use it as the uncertainty in position, delta x, of the electron in the Heisenberg Indeterminacy (Uncertainty) Equation.
Calculate 1% of the hydrogen radius (0.05 nm) in meters and use it as the uncertainty in position, delta x, of the electron in the Heisenberg Indeterminacy (Uncertainty) Equation.
Re: Uncertainty in Speed
I do not think we are covering this until week 2, and the week 2 homework can be from review or quantum, so maybe wait until Dr. Lavelle goes over this in lecture!
-
AnayaArnold_3L
- Posts: 56
- Joined: Wed Sep 18, 2019 12:19 am
Re: Uncertainty in Speed
Veronica_Lubera_1A wrote:The hydrogen atom has a radius of approximately 0.05 nm. Assume that we know the position of an electron to an accuracy of 1% of the hydrogen radius, calculate the uncertainty in the speed of the electron using the Heisenberg uncertainty principle.
So the correct answer is Delta V >= 10^8 m/s. How would you solve the question to receive this answer?
Dr.Lavelle will more than likely go over this concept more in a lecture during week 2 so I wouldn't worry about it too much as of right now.
-
KatherineValdez_4B
- Posts: 22
- Joined: Sat Sep 14, 2019 12:17 am
-
andrewcj 2C
- Posts: 102
- Joined: Thu Jul 11, 2019 12:17 am
Re: Uncertainty in Speed
I think it goes like this:
( p)(
p)( x) = (1/2)(h/2
x) = (1/2)(h/2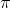 )
)
( p) = m(
p) = m( v)
v)
From here you can plug in (0.01)*(0.05 nm) for x and solve.
x and solve.
(
(
From here you can plug in (0.01)*(0.05 nm) for
-
andrewcj 2C
- Posts: 102
- Joined: Thu Jul 11, 2019 12:17 am
Re: Uncertainty in Speed
andrewcj 4I wrote:One additional thing for my reply, make sure you convert nm to m.
thanks for clarifying this.
Re: Uncertainty in Speed
andrewcj 4I wrote:One additional thing for my reply, make sure you convert nm to m.
ohh ok thank you!
-
KatherineValdez_4B
- Posts: 22
- Joined: Sat Sep 14, 2019 12:17 am
Re: Uncertainty in Speed
andrewcj 4I wrote:One additional thing for my reply, make sure you convert nm to m.
Thanks for this I almost forgot to do it.
Re: Uncertainty in Speed
Another important conceptual thing to think about is that the more you know about the position of an electron, the less we know about its velocity- they have an inverse relationship.
-
Anvi Brahmbhatt 4A
- Posts: 51
- Joined: Wed Sep 18, 2019 12:21 am
Re: Uncertainty in Speed
andrewcj 4I wrote:I think it goes like this:
(p)(
x) = (1/2)(h/2
)
(p) = m(
v)
From here you can plug in (0.01)*(0.05 nm) forx and solve.
Thank you for the explanation!
-
Claire Stoecklein 1E
- Posts: 49
- Joined: Sat Jul 20, 2019 12:15 am
Re: Uncertainty in Speed
When you determine delta p for the indeterminacy equation, make sure to use p=mv, using the velocity given in the equation and the mass of electron, 9.109 x 10^-31.
-
brennayoung
- Posts: 51
- Joined: Fri Sep 20, 2019 12:17 am
-
Ruth Glauber 1C
- Posts: 100
- Joined: Wed Sep 18, 2019 12:20 am
Re: Uncertainty in Speed
I think you need to calculate 1% of the hydrogen radius (0.05 nm) in meters for the uncertainty.
-
Caitlin Ciardelli 3E
- Posts: 44
- Joined: Wed Sep 18, 2019 12:19 am
Re: Uncertainty in Speed
Another way to calc uncertainty in v:
10 ms^-1 +/- .5
You can take the +/- value and multiply it by 2. This is another way to find uncertainty in v
10 ms^-1 +/- .5
You can take the +/- value and multiply it by 2. This is another way to find uncertainty in v
-
CMaduno_1L
- Posts: 102
- Joined: Wed Sep 18, 2019 12:18 am
Re: Uncertainty in Speed
In response to Brennayoung's question, I believe that we will only be applying this to hydrogen for now. This principle is used when measuring position and momentum of a particle.
-
Sara Richmond 2K
- Posts: 110
- Joined: Fri Aug 30, 2019 12:16 am
Re: Uncertainty in Speed
Use these equations:
(delta p)(delta x) = (1/2)(h/4 PIE)
(deltap) = m( delta v)
Sometimes this type of problem can be confusing because they dont give you the delta p equation.
Also remember to multiply the delta v uncertainty by 2 because it is plus or minus a certain amount.
(delta p)(delta x) = (1/2)(h/4 PIE)
(deltap) = m( delta v)
Sometimes this type of problem can be confusing because they dont give you the delta p equation.
Also remember to multiply the delta v uncertainty by 2 because it is plus or minus a certain amount.
-
Mitchell Koss 4G
- Posts: 128
- Joined: Sat Jul 20, 2019 12:17 am
-
Jack_Pearce_2H
- Posts: 110
- Joined: Wed Sep 30, 2020 9:48 pm
Re: Uncertainty in Speed
you would use the heisenberg uncertainty equations to solve this but more likely than not the result would be very off because the electron is known to a very close degree of certainty
Re: Uncertainty in Speed
If you need more help solving for these uncertainty questions this YouTube video really helped me get through these types of problems here is the link: https://www.youtube.com/watch?v=BNYz5EKXVeI
-
Harrington Bubb3A
- Posts: 50
- Joined: Fri Sep 24, 2021 6:29 am
Re: Uncertainty in Speed
KatherineValdez_4B wrote:I really hope he does cover it because I'm confused.
Right?! I thought this was a physics question at first and didn't even read through it until I realized it was a chem problem.
-
Harrington Bubb3A
- Posts: 50
- Joined: Fri Sep 24, 2021 6:29 am
Re: Uncertainty in Speed
Mitchell Koss 4G wrote:Also, don’t confuse frequency (v) with velocity (v)
I thought frequency was denoted with a lowercase f? Could be wrong that is based off of my physics class I took 3 years ago!
-
Jacob Kirkosian 3C
- Posts: 20
- Joined: Fri Sep 24, 2021 6:37 am
Re: Uncertainty in Speed
I believe that both a lowercase f and the Greek letter nu (which looks very similar to a v) can be used to denote frequency. However, nu is more often used when the frequency is related to electromagnetic waves.
Re: Uncertainty in Speed
Veronica_Lubera_2A wrote:The hydrogen atom has a radius of approximately 0.05 nm. Assume that we know the position of an electron to an accuracy of 1% of the hydrogen radius, calculate the uncertainty in the speed of the electron using the Heisenberg uncertainty principle.
So the correct answer is Delta V >= 10^8 m/s. How would you solve the question to receive this answer?
We will be covering this in weeks 2 and 3, you should wait until then to dive into these questions, its great that you are getting ahead though!
-
kareena_prasad
- Posts: 87
- Joined: Fri Sep 24, 2021 5:07 am
Re: Uncertainty in Speed
I think it is interesting how you can't determine the position and the speed of an electron at the same time. You can only find one if you have the other.
Re: Uncertainty in Speed
convert the radius into m and then multiply it by two to find the diameter which will act as your change in position (delta x). to find the mass of a hydrogen atom, convert the molar mass into kg/mol (SI units). you would take the molar mass, divide it by 1000 to convert into kg, and then use avogadro's number for the atoms. this acts as your mass. plug it all in the heisenberg equation and then solve for delta v (speed). note that momentum can also be written as mass times velocity so that's how you would solve for velocity.
Re: Uncertainty in Speed
convert the radius into m and then multiply it by two to find the diameter which will act as your change in position (delta x). to find the mass of a hydrogen atom, convert the molar mass into kg/mol (SI units). you would take the molar mass, divide it by 1000 to convert into kg, and then use avogadro's number for the atoms. this acts as your mass. plug it all in the heisenberg equation and then solve for delta v (speed). note that momentum can also be written as mass times velocity so that's how you would solve for velocity.
-
Joellen 1B
- Posts: 104
- Joined: Fri Sep 24, 2021 6:24 am
Re: Uncertainty in Speed
I would use the Heisenberg uncertainty equation which is the uncertainty in momentum multiplied by the uncertainty in position is greater than or equal to planck's constant over 4pi. The uncertainty in momentum is equal to mass times the uncertainty in velocity, so you could use that in solving. But overall, I would remember that the uncertainty in position would be the radius you were given multiplied by two to get the diameter.
-
kayleec1004
- Posts: 74
- Joined: Fri Sep 24, 2021 6:57 am
-
Sophia Hartwell 1F
- Posts: 65
- Joined: Fri Sep 24, 2021 5:42 am
Re: Uncertainty in Speed
I'm confused about this as well but based on the syllabus this should be covered in week 2 so hopefully he will go over it then.
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 2 guests

