For Textbook Question 1B.25, how do would you go about finding the speed of the electron? I understand that the end goal is finding the v from change in momentum, but how do we find change in position in the Heisenberg Equation? Is it from the diameter of the lead atom?
Any explanation may help, thanks!
Textbook 1B 25
Moderators: Chem_Mod, Chem_Admin
-
Pranav Daggubati 3C
- Posts: 121
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Textbook 1B 25
Correct, you would use the diameter of the atom as 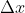 because that is the boundary of the electron is space.
because that is the boundary of the electron is space.
-
KatarinaReid_3H
- Posts: 204
- Joined: Wed Sep 30, 2020 9:41 pm
- Been upvoted: 4 times
Re: Textbook 1B 25
First, use the equation delta p is greater or equal to (h/(4pi(delta x)).
Delta x = 350e-12 meters. Now solve for p(momentum). Once you have p=1.49e-24, then set that equal to mass x velocity. You have the mass of an electron, so you just need to solve for velocity which is 1.63e5 m/s.
Delta x = 350e-12 meters. Now solve for p(momentum). Once you have p=1.49e-24, then set that equal to mass x velocity. You have the mass of an electron, so you just need to solve for velocity which is 1.63e5 m/s.
-
EmilyGillen_1A
- Posts: 124
- Joined: Wed Sep 30, 2020 9:49 pm
- Been upvoted: 1 time
Re: Textbook 1B 25
For this problem I follow the logic and step/process, but I am confused as to why the delta(v) is only 5 instead of 10. In the equation it says that the speed is 5.0 m/s + or - 5, so wouldn't that mean the uncertainty is 10 not 5? (5 for +5 and 5 for -5)
I guess I am confused because I thought in lecture he mentioned making sure to count both side of the given uncertainty, but putting 10 in here would give you the wrong answer.
I guess I am confused because I thought in lecture he mentioned making sure to count both side of the given uncertainty, but putting 10 in here would give you the wrong answer.
-
Asia Yamada 2B
- Posts: 102
- Joined: Wed Sep 30, 2020 9:36 pm
Re: Textbook 1B 25
EmilyGillen_1A wrote:For this problem I follow the logic and step/process, but I am confused as to why the delta(v) is only 5 instead of 10. In the equation it says that the speed is 5.0 m/s + or - 5, so wouldn't that mean the uncertainty is 10 not 5? (5 for +5 and 5 for -5)
I guess I am confused because I thought in lecture he mentioned making sure to count both side of the given uncertainty, but putting 10 in here would give you the wrong answer.
I think you're referring to 1B 27, but yes, the uncertainty in velocity would be 10 because you multiply by two when there’s a ± because that represents the range of values that the speed could be. I believe that there’s an error in the answer key. You can find the correct solution here. https://lavelle.chem.ucla.edu/wp-conten ... rs_7Ed.pdf
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 8 guests

