The hydrogen atom has a radius of approximately 0.05 nm. Assume that we know the position of an electron to an accuracy of 1 % of the hydrogen radius, calculate the uncertainty in the speed of the electron using the Heisenberg uncertainty principle.
How would I go about solving this? I know how to use the equation but i think the radius and 1% is throwing me off. Thank you
heisenberg module #18
Moderators: Chem_Mod, Chem_Admin
-
John Pham 3L
- Posts: 149
- Joined: Wed Sep 30, 2020 9:49 pm
- Been upvoted: 4 times
Re: heisenberg module #18
For uncertainty in position, they tell you that it is 1% of the Hydrogen atom's radius.
To find this, just multiply 0.05 nm by 0.01 and convert to m for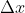
Once you have this, plug it into the Heisenberg Indeterminacy equation and solve for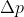
Remember that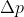 equals
equals 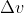 times mass of electron (9.11*10^-31 kg).
times mass of electron (9.11*10^-31 kg).
Solve for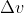 to get the uncertainty in the speed of the electron
to get the uncertainty in the speed of the electron
To find this, just multiply 0.05 nm by 0.01 and convert to m for
Once you have this, plug it into the Heisenberg Indeterminacy equation and solve for
Remember that
Solve for
-
Tessa House 3A
- Posts: 101
- Joined: Wed Sep 30, 2020 9:52 pm
- Been upvoted: 1 time
Re: heisenberg module #18
Since they give you the radius and say the uncertainty in position is 1% of this value, you can find uncertainty in position first. Convert 0.05 nm to m because these units are needed for later calculations. Then take 1% of this value by multiplying it by 0.01 to find the uncertainty in position. From there, you can use Heisenberg's indeterminacy principle to solve for delta p using delta x. Then, calculate delta v, uncertainty in speed, using delta p calculated before and the mass of an electron, a constant given to you. This is your answer.
-
Asia Yamada 2B
- Posts: 102
- Joined: Wed Sep 30, 2020 9:36 pm
Re: heisenberg module #18
First, you would convert the radius into meters which would be 5x10^-11m. You have to multiply the radius by 0.01 because the radius is known to an accuracy of 1%. You should get 5x10^-13m. This is the uncertainty in position and from there you can just plug the uncertainty in position into Heisenberg’s Indeterminacy Equation (Δx • Δp ≥ h/4pi). This will give you the uncertainty in momentum. Since p=mv, and you know the mass of an electron (9.109x10^-31kg), you can solve for uncertainty in velocity.
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 2 guests

