The equation
Moderators: Chem_Mod, Chem_Admin
-
jordanginyard_
- Posts: 101
- Joined: Wed Sep 30, 2020 9:43 pm
The equation
When do we know we have to use the Heisenberg equation? I get confused when we use it, is it when it asking for the translation, for example, n=5 to n=2.
-
Ryan_Page_1J
- Posts: 100
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 1 time
Re: The equation
The Heisenberg equation relates to indeterminacy in position or momentum of objects. I think you are thinking of the Rydberg equation.
-
Sunny Wu 3A
- Posts: 139
- Joined: Wed Sep 30, 2020 9:58 pm
- Been upvoted: 1 time
Re: The equation
The Heisenberg Uncertainty Equation states that there is a limit on the accuracy to which the momentum and position of a particle can be known simultaneously. The equation is as follows:
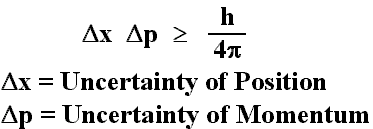
You might be confusing it with the Rydberg Equation, which gives you the frequency/wavelength of the energy released when an electron in a hydrogen atom goes from one energy level (in your example n=5) to a lower energy level (n=2).

In this equation, n=2 would be n1 and n=5 would be n2
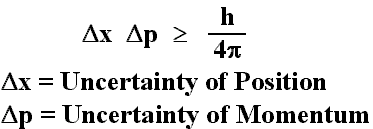
You might be confusing it with the Rydberg Equation, which gives you the frequency/wavelength of the energy released when an electron in a hydrogen atom goes from one energy level (in your example n=5) to a lower energy level (n=2).

In this equation, n=2 would be n1 and n=5 would be n2
Last edited by Sunny Wu 3A on Sun Nov 01, 2020 2:39 pm, edited 1 time in total.
-
manisha_joseph_1H
- Posts: 101
- Joined: Wed Sep 30, 2020 10:01 pm
- Been upvoted: 1 time
Re: The equation
The Heisenberg Uncertainty equation should be used when dealing with a problem that includes the components of position (such as the diameter of an atom), mass, and velocity (mv = momentum (p). Moreover, a question will most likely ask you to determine the uncertainty in either the position, velocity, or momentum altogether, so just be able to recognize that this will correspond with using the Heisenberg Uncertainty equation.
-
Arti_Patel_3H
- Posts: 107
- Joined: Wed Sep 30, 2020 9:38 pm
Re: The equation
If you're confused about Rydberg's, know that if the photon is emitted, n (initial) should be greater than n (final). If the photon is absorbed, it is the opposite.
-
Keshav Patel 14B 2B
- Posts: 104
- Joined: Wed Sep 30, 2020 9:40 pm
Re: The equation
Any time you have a question that either gives or asks for the indeterminacy for velocity or position, then you know you will be using the Heisenberg Equation.
Re: The equation
We know we have to use the Heisenberg equation when we are asked for uncertainty in momentum or position. When given states of an electron we would probably be asked to use the Rydberg equation, like you described.
-
Mauricio Maravilla 3C
- Posts: 50
- Joined: Wed Sep 30, 2020 9:33 pm
Re: The equation
I have another question, if a problem gives us the speed for example of an electron, we can get the momentum since we know what the mass of an electron is. Do we then multiply delta x by the momentum, since the uncertainty in its momentum is 100% which is just one?
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 6 guests

