Hi everyone!
Could you guys explain to me the overall concept of the Heisenberg Interdeterminacy Principle? I understand how to calculate a Heisenberg problem when I see it, but I don't really understand the concept behind it.
Thank you so much!
Heisenberg Indeterminacy Concept
Moderators: Chem_Mod, Chem_Admin
-
Sid Panda 3A
- Posts: 150
- Joined: Wed Sep 30, 2020 9:58 pm
- Been upvoted: 1 time
Re: Heisenberg Indeterminacy Concept
Stella Nguyen DIS 1J wrote:Hi everyone!
Could you guys explain to me the overall concept of the Heisenberg Interdeterminacy Principle? I understand how to calculate a Heisenberg problem when I see it, but I don't really understand the concept behind it.
Thank you so much!
When particles start to get extremely small, it is a lot harder to pinpoint what their position/momentum could be to a certain amount of accuracy. For example, a very large object like a car would be very easy to measure precisely; we can determine the momentum of the car if we know it's mass + velocity, and we can also determine the position of the car. On the other hand, an electron would be very difficult to determine it's location or momentum just because of how small it is. Heisenberg Interdeterminacy Principle is just a formula that can be used to calculate what amount of certainty we can determine the momentum or position of an atom to have.
-
Elizabeth Kim 2K
- Posts: 50
- Joined: Fri Sep 24, 2021 5:11 am
Re: Heisenberg Indeterminacy Concept
The Heisenberg Uncertainty Principle states that you cannot simultaneously know the exact values of both position and momentum of a particle. The more precisely the position of a particle is known, the more uncertain the momentum is, and vice versa.
-
Darren Apostol 2L
- Posts: 102
- Joined: Fri Sep 24, 2021 5:12 am
- Been upvoted: 1 time
Re: Heisenberg Indeterminacy Concept
When we take a measurement, we interact with the object using light (or electrons). Both have momentum. When we take a measurement, we shine light on the object, and the momentum of the photons is transferred to the object. For large, macroscopic objects, the transfer is insignificant, but for microscopic objects, like electrons, that transferred momentum is significant.
From de Broglie's equation,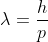 , the shorter the wavelength, the higher the momentum. For things as small as electrons, we need to use very low wavelength light, which means very high momentum. The more precisely we want to know the position, the lower the wavelength we need to use, and the higher the momentum transferred. Thus, there is more uncertainty in the momentum of the object. As a side note, if we use higher wavelength light, we lose resolution (accuracy), so while we won't affect the momentum as much, we will know less of the position.
, the shorter the wavelength, the higher the momentum. For things as small as electrons, we need to use very low wavelength light, which means very high momentum. The more precisely we want to know the position, the lower the wavelength we need to use, and the higher the momentum transferred. Thus, there is more uncertainty in the momentum of the object. As a side note, if we use higher wavelength light, we lose resolution (accuracy), so while we won't affect the momentum as much, we will know less of the position.
From de Broglie's equation,
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 8 guests

