The question asks, "What would the speed of each particle be if it had the same wavelength as a photon of red light (lambda=750.0 nm)?"
I was thinking of using an equation to find energy first, and then solving for velocity using another equation, but I'm not sure if that would be correct. Would speed and velocity be interchangeable in this situation?
Achieve Question 14
Moderators: Chem_Mod, Chem_Admin
-
Jocelyn Chin 1K
- Posts: 104
- Joined: Fri Sep 24, 2021 5:12 am
- Been upvoted: 1 time
Re: Achieve Question 14
Velocity is just speed but with a direction, so you are right, they would be interchangeable! I used DeBroglie's equation because it included lambda and velocity in one equation, which I thought would be more simple for calculations. I set lambda = h/p = h/(mv). Then I plugged in the photon wavelength and solved for v. I hope this helps!
-
MaiVyDang2I
- Posts: 104
- Joined: Fri Sep 24, 2021 6:52 am
Re: Achieve Question 14
For this question, I'd use the equation wavelength = h/p = h/(mv) and plug all values in to find speed instead of finding energy first. I'm not sure how to find speed after finding energy because the kinetic energy equation doesn't work.
-
Adithi Ayyala 2G
- Posts: 105
- Joined: Fri Sep 24, 2021 6:05 am
- Been upvoted: 1 time
Re: Achieve Question 14
Hi everyone! Okay, so what worked is first, using the wavelenght= h/p equation. p here represents mass in kg x velocity. Thus, this equation (wavelength= h/(mass x velocity)) can be manipulated to say "velocity= h/(mass x wavelength)". The wavelength should be converted from nm to m.
You would plug in all three values (h=6.626x10^-34 Js) and yield the velocity in m/s.
I hope this helps!!
You would plug in all three values (h=6.626x10^-34 Js) and yield the velocity in m/s.
I hope this helps!!
-
Andrew Nguyen 1E
- Posts: 101
- Joined: Fri Sep 24, 2021 6:34 am
- Been upvoted: 1 time
Re: Achieve Question 14
For this problem, I found velocity using 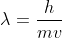 (de Broglie's equation) for each given particle. I don't think you need to find the energy of anything in this case. We cannot even use
(de Broglie's equation) for each given particle. I don't think you need to find the energy of anything in this case. We cannot even use 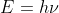 since all the particles have a mass.
since all the particles have a mass.
Also, speed can be considered the same thing as velocity for our purposes.
Also, speed can be considered the same thing as velocity for our purposes.
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 5 guests

