A proton is accelerated in a cyclotron to a very high speed that is known to within 3.0x10^2 km.s^-1. What is the minimum uncertainty in its position?
How would I solve this question?
Question from the textbook
Moderators: Chem_Mod, Chem_Admin
-
Nancy Li 1C
- Posts: 104
- Joined: Fri Sep 24, 2021 6:46 am
Re: Question from the textbook
We are given that ∆v = 3.0 x 10^2 km/s so I would first convert this to m/s (3.0 x 10^5 m/s). Using Heisenberg's Uncertainty Equation, we have that ∆x∆p >= h/4pi. ∆p = m∆v so using the mass of a proton we find that ∆p = 1.67 x 10^-27 * 3.0 x 10^5 = 5.01 x 10^-22. Plugging this back into Heisenberg's Equation, we are able to solve for ∆x for a final answer of 1.05 x 10^-13 m.
-
Sidney Shah 3H
- Posts: 100
- Joined: Fri Sep 24, 2021 5:47 am
- Been upvoted: 1 time
Re: Question from the textbook
The value given, 3.0x10^2 km.s^-1, is equal to delta v, which you can use to solve for delta p or momentum by multiplying by the mass of a proton. Then you can plug this value into the Heisenberg Indeterminacy Equation!
-
Amanda Tran 1D
- Posts: 101
- Joined: Fri Sep 24, 2021 7:10 am
- Been upvoted: 1 time
Re: Question from the textbook
The problem gives you the value for 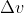 , and is asking you to solve for
, and is asking you to solve for 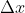 . Using Heisenberg's uncertainty principle, we know that
. Using Heisenberg's uncertainty principle, we know that 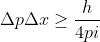 , which can also be written as
, which can also be written as 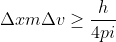 . We want to solve for
. We want to solve for 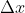 , so we can rearrange this to look like:
, so we can rearrange this to look like: 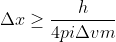 . From here, you can plug in the value for delta V which you were given, and then Planck's constant and the mass of the proton.
. From here, you can plug in the value for delta V which you were given, and then Planck's constant and the mass of the proton.
-
Harrington Bubb3A
- Posts: 50
- Joined: Fri Sep 24, 2021 6:29 am
Re: Question from the textbook
If you know the velocity, then solve for the change in x by dividing the right hand of the equation by the velocity and you get the displacement. Make sure to convert to SI units!
Return to “Heisenberg Indeterminacy (Uncertainty) Equation”
Who is online
Users browsing this forum: No registered users and 2 guests

