Atom Spin
Moderators: Chem_Mod, Chem_Admin
-
sambrown12
- Posts: 82
- Joined: Fri Sep 29, 2023 11:04 am
Atom Spin
Hi! How do we tell the difference between whether or not the atom is spinning forward or backwards. In other words how can we tell that the ml is +1/2 or -1/2?
-
Gabrielle Arreglo 3F
- Posts: 122
- Joined: Fri Sep 29, 2023 11:08 am
Re: Atom Spin
*ml is the magnetic quantum number. ms is for spin and ms can be +1/2 or -1/2
The way electron spin was discovered was through the Stern-Gerlach experiment, where they sent silver particles through a magnetic field and there were two different beams. You need complex equipment to tell whether the electron spin is up or down, but if you're given the number of electrons & the electron configuration, you can tell whether an electron has up or down spin based on Hund's rule.
Ex: Does is the 6th electron of Carbon up or down?
2p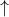 .
. 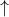
2s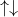
1s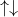
The 6th electron of Carbon has an up spin because the electrons must fill all the possible orbitals with parallel spins before adding the opposite spins. In other words, there should be up arrows in every orbital before you add pair them with the down arrows.
The way electron spin was discovered was through the Stern-Gerlach experiment, where they sent silver particles through a magnetic field and there were two different beams. You need complex equipment to tell whether the electron spin is up or down, but if you're given the number of electrons & the electron configuration, you can tell whether an electron has up or down spin based on Hund's rule.
Ex: Does is the 6th electron of Carbon up or down?
2p
2s
1s
The 6th electron of Carbon has an up spin because the electrons must fill all the possible orbitals with parallel spins before adding the opposite spins. In other words, there should be up arrows in every orbital before you add pair them with the down arrows.
-
Rebecca Kam 3D
- Posts: 40
- Joined: Thu Oct 05, 2023 8:22 am
Re: Atom Spin
For me, it helps to write it out to see which orbitals are being filled. But you can tell when ms is spinning up or down based on the number of electrons and which orbital you are in. From the Pauli Exclusion Principle, there can only be a maximum of two electrons per orbital, and these electrons need to be in a spin direction opposite of each other. So, this is why one is + and one is - for the 1/2. With Hund's rule, electrons in the orbital need to be filled with electrons that have spins parallel to each other. An example of this is sodium which has the electron configuration of [Ne] 3s^1. If you draw this out, the 3s will have one orbital that is filled with one electron and this will be spinning up because it is +1/2 and the first arrows should be up.
-
Averie Moore 2F
- Posts: 79
- Joined: Fri Sep 29, 2023 11:28 am
Re: Atom Spin
When you are drawing the diagram for the filling of these orbitals, the arrows are inputted from left to right and only once the orbital has been filled can they be paired with an electron spinning in the opposite direction.
Return to “Wave Functions and s-, p-, d-, f- Orbitals”
Who is online
Users browsing this forum: No registered users and 5 guests

