D orbital
Moderators: Chem_Mod, Chem_Admin
Re: D orbital
Yes. There are five orbitals, which is of the nature of the D orbital, as there are three p orbitals and one S orbital. Each orbital holds two electrons, so the D orbitals have the capability to hold 10 electrons
-
Michelle Le 1J
- Posts: 50
- Joined: Fri Aug 09, 2019 12:16 am
Re: D orbital
If you look at it using the quantum numbers, the D subshell is l=2, therefore ml (the orbitals) is 2,1,0,-1,-2. There are 5 values, meaning that there is a total of 5 orbitals in the D subshell.
-
Jialun Chen 4F
- Posts: 108
- Joined: Sat Sep 07, 2019 12:16 am
Re: D orbital
Since each orbital holds two electrons, the d-orbital has 5, allowing for ten total electrons.
-
ValerieChavarin 4F
- Posts: 99
- Joined: Wed Sep 18, 2019 12:18 am
Re: D orbital
If you take a look at the magnetic quantum numbers 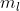 for the d, they are -2, -1, 0, +1, +2, which are 5 in total. Each orbital is able to hold 2 electrons, therefore the d-orbital can hold a total of 10 electrons.
for the d, they are -2, -1, 0, +1, +2, which are 5 in total. Each orbital is able to hold 2 electrons, therefore the d-orbital can hold a total of 10 electrons.
Return to “Wave Functions and s-, p-, d-, f- Orbitals”
Who is online
Users browsing this forum: No registered users and 9 guests

