Definition and Formula
Moderators: Chem_Mod, Chem_Admin
-
Irene Kang 3F
- Posts: 102
- Joined: Fri Sep 24, 2021 7:04 am
- Been upvoted: 1 time
Definition and Formula
Can anyone give me a different definition of wavefunction that tells me why we use it? What is the actual formula to find the wavefunction? What is the difference between the wavefunction based on the spherical polar coordinates(1.D2) and the wavefunction from Schrodinger's equation(Hwavefunction=Ewavefunction)? How can we find the radial wavefunction and the angular wavefunction?
-
Paul Zhang 2F
- Posts: 55
- Joined: Fri Sep 24, 2021 7:35 am
- Been upvoted: 1 time
Re: Definition and Formula
Hello! The actual wave function is horrendously complicated, which is probably why we're not taught the whole thing. I think the Chem 20A class goes more in detail. For example, they use the simplified model of a "particle in a box." A particle is allowed to move horizontally within a "well" of zero potential energy with regions of infinite potential energy (so the particle can't escape). Then the time-independent Schrodinger equation (}{dx^2} + V(x)\psi(x) = E\psi(x)) ) can be solved to find the wavefunction
) can be solved to find the wavefunction  = A\sin(kx) + B\cos(kx)) . Since the particle can only be in the well, through some math the wavefunction can be simplified to
. Since the particle can only be in the well, through some math the wavefunction can be simplified to  = A\sin(kx)) . Solving for the constants A and k gives
. Solving for the constants A and k gives 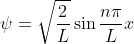 . Solving for the energy gives
. Solving for the energy gives 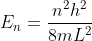 . There's a lot more detail in https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/05.5%3A_Particle_in_Boxes/Particle_in_a_1-Dimensional_box.
. There's a lot more detail in https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/05.5%3A_Particle_in_Boxes/Particle_in_a_1-Dimensional_box.
Couple interesting things: The equation for energy shows that it's discrete and that the energy can never be zero. Also, as the energy level increases,
increases,  looks more and more like a line, which is why everyday objects (which have a lot more energy than atomic particles) don't show wavelike properties. The wavefunction can be squared to find the probability, which is useful because it ensures the amplitude of the wave is made positive (can't have negative probability). Why is it squared? I'm pretty sure that was experimentally determined: (https://arxiv.org/abs/0811.2068).
looks more and more like a line, which is why everyday objects (which have a lot more energy than atomic particles) don't show wavelike properties. The wavefunction can be squared to find the probability, which is useful because it ensures the amplitude of the wave is made positive (can't have negative probability). Why is it squared? I'm pretty sure that was experimentally determined: (https://arxiv.org/abs/0811.2068).
By the way, using spherical coordinates (along with the radial and angular stuff) is helpful in solving the Schrodinger equation using separation of variables. Hopefully that's helpful! This is all just based on stuff I read, so apologies if it's incorrect or unclear.
Couple interesting things: The equation for energy shows that it's discrete and that the energy can never be zero. Also, as the energy level
By the way, using spherical coordinates (along with the radial and angular stuff) is helpful in solving the Schrodinger equation using separation of variables. Hopefully that's helpful! This is all just based on stuff I read, so apologies if it's incorrect or unclear.
Return to “Wave Functions and s-, p-, d-, f- Orbitals”
Who is online
Users browsing this forum: No registered users and 5 guests

