Quantum Numbers
Moderators: Chem_Mod, Chem_Admin
-
Jonathan Shyu 3L
- Posts: 102
- Joined: Fri Sep 24, 2021 5:07 am
Quantum Numbers
So from Schrodinger's Wave Equation, the three quantum numbers are n, l, and Ml. Letter n corresponds to the energy level or electron level, l refers to the shape (s,p,d,f), and Ml refers to the orientation. However, I'm not quite sure what it means by orientation. So if Ml=1, what does that mean?
-
Grace Chang 1E
- Posts: 103
- Joined: Fri Sep 24, 2021 6:54 am
- Been upvoted: 3 times
Re: Quantum Numbers
Hi Jonathan!
Ml=1 can mean various things depending on what the l quantum number is. However, it should be noted that the only ml quantum number for the "s" sub shell is zero—this is because its only orientation is a sphere-like ball.
However, for the p sub shell, there are 3 possible orientations: px, py, and pz. Here's a link to what they look like: https://s3-us-west-2.amazonaws.com/cour ... 424730.png
In Dr. Lavelle's examples, he assigned px to ml=1, but I believe he mentioned that others label it in the opposite order (like ml=1 corresponds to pz instead).
For the d-block, there are 5 different possible arrangements of the electron density (shown in lecture). Each ml would then correspond to one of these unique arrangements.
I hope that helps!!:)
Ml=1 can mean various things depending on what the l quantum number is. However, it should be noted that the only ml quantum number for the "s" sub shell is zero—this is because its only orientation is a sphere-like ball.
However, for the p sub shell, there are 3 possible orientations: px, py, and pz. Here's a link to what they look like: https://s3-us-west-2.amazonaws.com/cour ... 424730.png
In Dr. Lavelle's examples, he assigned px to ml=1, but I believe he mentioned that others label it in the opposite order (like ml=1 corresponds to pz instead).
For the d-block, there are 5 different possible arrangements of the electron density (shown in lecture). Each ml would then correspond to one of these unique arrangements.
I hope that helps!!:)
-
Maxwell Yao
- Posts: 102
- Joined: Fri Sep 24, 2021 5:38 am
Re: Quantum Numbers
Adding to what Grace said, the range of numbers possible for 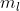 is 0
is 0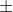 l.
l.
For example, if we're talking about 2p , n = 2, l = 1, and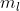 could be -1, 0 or 1 where each of those three numbers represents either the px, py or pz orbitals.
could be -1, 0 or 1 where each of those three numbers represents either the px, py or pz orbitals.
Hope this helps.
For example, if we're talking about 2p , n = 2, l = 1, and
Hope this helps.
Return to “Wave Functions and s-, p-, d-, f- Orbitals”
Who is online
Users browsing this forum: No registered users and 2 guests

