Homework question on 25
Moderators: Chem_Mod, Chem_Admin
-
Anisa Morales 1L
- Posts: 104
- Joined: Fri Sep 24, 2021 7:36 am
Homework question on 25
Hello,
I think I understand the question however I was wondering what the [Ne] referred to. Like why is it there>
Question: " Give the chemical symbol for the element with the ground‑state electron configuration [Ne]3s^2 3p^2 ."
I think I understand the question however I was wondering what the [Ne] referred to. Like why is it there>
Question: " Give the chemical symbol for the element with the ground‑state electron configuration [Ne]3s^2 3p^2 ."
-
Elaine Steinberg 3H
- Posts: 105
- Joined: Fri Sep 24, 2021 6:47 am
Re: Homework question on 25
Instead of writing out the whole entire electron configuration for Si (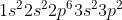 ) or any other element, you can use an abbreviated notation. The part of the electron configuration that represents completely filled shells is replaced with the noble gas it corresponds to in brackets (in this case
) or any other element, you can use an abbreviated notation. The part of the electron configuration that represents completely filled shells is replaced with the noble gas it corresponds to in brackets (in this case 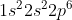 is the configuration for Ne). Then, the outer electrons that don't compromise a full subshell are written after the brackets (the ones leftover -- in this case
is the configuration for Ne). Then, the outer electrons that don't compromise a full subshell are written after the brackets (the ones leftover -- in this case 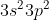 ). Together, this makes [Ne]
). Together, this makes [Ne]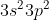 .
.
-
Anisa Morales 1L
- Posts: 104
- Joined: Fri Sep 24, 2021 7:36 am
Re: Homework question on 25
Elaine Steinberg 1H wrote:Instead of writing out the whole entire electron configuration for Si () or any other element, you can use an abbreviated notation. The part of the electron configuration that represents completely filled shells is replaced with the noble gas it corresponds to in brackets (in this case
is the configuration for Ne). Then, the outer electrons that don't compromise a full subshell are written after the brackets (the ones leftover -- in this case
). Together, this makes [Ne]
.
are we required to do this for the test/ in general?
-
Hannah Joo 2D
- Posts: 101
- Joined: Thu Oct 07, 2021 5:05 am
Re: Homework question on 25
In general, it isn't required scientifically for you to use the "shortcut" but it is the norm because it is tedious to always have to write out everything especially when dealing with elements that have higher atomic numbers.
-
masontsang
- Posts: 52
- Joined: Fri Sep 24, 2021 5:09 am
Re: Homework question on 25
Hi!
The [Ne] represents the electron configuration of Neon and is used as a shortcut or abbreviation for another element's electron configuration. You can only use the abbreviation for the closest noble gas (group 18 elements) to your element because noble gases have completely filled shells. After using the abbreviated version, you still need to write out the orbitals of the uncompleted subshell(s)!
I hope this helps!
The [Ne] represents the electron configuration of Neon and is used as a shortcut or abbreviation for another element's electron configuration. You can only use the abbreviation for the closest noble gas (group 18 elements) to your element because noble gases have completely filled shells. After using the abbreviated version, you still need to write out the orbitals of the uncompleted subshell(s)!
I hope this helps!
-
Madelyn_Rios_2c
- Posts: 110
- Joined: Fri Sep 24, 2021 5:54 am
Re: Homework question on 25
The [Ne] refers to the electron configuration of Neon and the abbreviation is used as a shortcut for the electron configuration of another element. For Neon, the configuration is 1s22s22p6.
-
Celine Khuu 2F
- Posts: 100
- Joined: Fri Sep 24, 2021 6:31 am
Re: Homework question on 25
The way that the problem is doing electron configuration is the shorthand way! [Ne] replaces the 1s^2 2s^2 2p^6 in the electron configuration of the element in question.
-
Katryn Heine 3A
- Posts: 109
- Joined: Fri Sep 24, 2021 7:18 am
- Been upvoted: 1 time
Re: Homework question on 25
The [Ne] is something referred to as noble gas configurations and they're basically a short cut to writing out the electron configurations. so instead of writing out the full first few configurations you can just put the noble gas that represents those configurations first and then add the addition configurations afterwards.
-
madeleinewright
- Posts: 54
- Joined: Fri Sep 24, 2021 5:36 am
Re: Homework question on 25
The configuration with [Ne] in the beginning is the noble gas configuration for the element. Instead of writing out the entire ground-state electron configuration, you can write the most recent noble gas for the element and then write the ground-state electron configuration for the element following that noble gas.
-
Thailer Phorn 1C
- Posts: 99
- Joined: Wed Nov 25, 2020 12:20 am
Re: Homework question on 25
Using the [Ne] is just a shortcut so that you do not have to write out the entire electron configuration. And I think that on a test it would be specified if we have to write out the entire configuration.
-
Ginny Ghang 1B
- Posts: 102
- Joined: Fri Sep 24, 2021 7:22 am
Re: Homework question on 25
[Ne] is just a shortcut or shorthand to make it easier when writing the electron configuration. You can use the noble gas elements, put them in brackets, and write out the last line of the electron configuration to make it shorter and easier to write. However, if it asks for the complete configuration, you have to write out the entire thing out without using the shortcut.
-
Zoe Dhalla 3I
- Posts: 104
- Joined: Fri Sep 24, 2021 5:44 am
Re: Homework question on 25
Hi
So basically, that is the shorthand (or noble gas notation) of an element. To write in noble gas notation, simply start with the symbol of the noble gas in the previous period in brackets (like [Ne] for example), followed by the additional configuration of the electrons for the given element So in this situation, The notation [Ne] represents the core electron configuration, 1s^22s^22p^6, which is another way to say 10 electrons.
So, to solve this problem you can add 10 to the electrons given after the noble gas (because neon has 10 electrons), or write out the full configuration 1s^22s^22p^63s^2 3p^2 and solve normally.
Hope this helps!
So basically, that is the shorthand (or noble gas notation) of an element. To write in noble gas notation, simply start with the symbol of the noble gas in the previous period in brackets (like [Ne] for example), followed by the additional configuration of the electrons for the given element So in this situation, The notation [Ne] represents the core electron configuration, 1s^22s^22p^6, which is another way to say 10 electrons.
So, to solve this problem you can add 10 to the electrons given after the noble gas (because neon has 10 electrons), or write out the full configuration 1s^22s^22p^63s^2 3p^2 and solve normally.
Hope this helps!
-
Tyler Olson 1E
- Posts: 85
- Joined: Fri Sep 24, 2021 5:40 am
Re: Homework question on 25
Having the [Ne] there makes it simpler to write out. It is just a shortcut rather than writing out the entire configuration. These can't be done with every element, only the noble gasses and unless a question is asking for the complete configuration it is easier to write it out in that way. Also by looking at the noble gas you can get the full configuration by writing out that of the noble gas and then adding the other electrons listed.
-
Michelle Jeong 1B
- Posts: 102
- Joined: Fri Sep 24, 2021 6:49 am
Re: Homework question on 25
That's a short cut basically. Instead of writing out the whole electron configuration you can just start from the preceding noble gas.
Re: Homework question on 25
Hi,
I think you could understand [Ne] as what the electron configuration of the atom X already owned. In this case, X should be at the next row of that where Ne is at. For [X], you could just write [Ne] plus specific electronic configurations after it for the sake of convenience.
For example, Na has an additional electron on the 3s orbital compared to Ne. In this case, you could directly write [Na]=[Ne]3s1, instead of [Na]=1s22s22p63s1.
I hope this could address your concerns.
I think you could understand [Ne] as what the electron configuration of the atom X already owned. In this case, X should be at the next row of that where Ne is at. For [X], you could just write [Ne] plus specific electronic configurations after it for the sake of convenience.
For example, Na has an additional electron on the 3s orbital compared to Ne. In this case, you could directly write [Na]=[Ne]3s1, instead of [Na]=1s22s22p63s1.
I hope this could address your concerns.
Last edited by 305805394 on Sun Oct 24, 2021 10:08 pm, edited 1 time in total.
-
Rishab_Haldar_3B
- Posts: 112
- Joined: Fri Sep 24, 2021 7:26 am
Re: Homework question on 25
The symbol [Ne] refers to the electron configuration of the last noble gas where all of its shells are completely filled. The electrons that come after [Ne] are the electrons that are not part of a completely filled shell.
-
Hannah Thornton 1F
- Posts: 104
- Joined: Fri Sep 24, 2021 5:53 am
Re: Homework question on 25
using an element in the [ ] brackets represents an abbreviated notation for its electron configuration. since part of the other atom's electron configuration is the same as the bracketed element, it makes it much simpler to represent that section of the configuration with the element's name.
Re: Homework question on 25
Using other elements is a form of short hand to make it easier to write out these configurations. it’s basically that many electrons plus whatever follows that element, so all you have to do is add the amount of electrons in that element to the rest to find what the configuration is for.
-
Wenhan Li_3d
- Posts: 50
- Joined: Wed Feb 17, 2021 12:15 am
Re: Homework question on 25
Ne is used as abbreviation and a shortcut when writing the electronic configuration because there are some configuration is really long and not necessary to write them all. But for some questions you should consider writing the full configuration based on what the question asked.
Return to “Wave Functions and s-, p-, d-, f- Orbitals”
Who is online
Users browsing this forum: No registered users and 3 guests

