Hund's Rule vs Pauli Exclusion Principle
Moderators: Chem_Mod, Chem_Admin
Hund's Rule vs Pauli Exclusion Principle
What is the difference between Hund's Rule and the Pauli Exclusion principle?
-
Lindsay Beckwith 2B
- Posts: 122
- Joined: Wed Feb 10, 2021 12:15 am
- Been upvoted: 1 time
Re: Hund's Rule vs Pauli Exclusion Principle
Hund's Rule is what explains why electrons will be unpaired/why there will be empty orbitals in an energy level. It states that if 2 or more orbitals are available within the same energy level, one electron goes into each one until they all have one elecron, and then each orbital is filled with a second electron as needed (according to the element).
Pauli Exclusion Principle simply states that every electron can be identified by its own unique set of quantum numbers, and that no two electrons can have the same set of quantum numbers. If you want to apply this to electrons within the same energy level/orbital, this principle also means that electrons occupying the same orbital must "spin" in different directions.
Pauli Exclusion Principle simply states that every electron can be identified by its own unique set of quantum numbers, and that no two electrons can have the same set of quantum numbers. If you want to apply this to electrons within the same energy level/orbital, this principle also means that electrons occupying the same orbital must "spin" in different directions.
-
Ethan Hung 2A
- Posts: 103
- Joined: Fri Sep 24, 2021 6:02 am
Re: Hund's Rule vs Pauli Exclusion Principle
Hund's Rule refers to the way in which electrons will fill up orbitals in the subshell; electrons with a spin that is parallel have a lower energy and are thus more stable than electrons that are paired. As a result, you first add one unpaired to each electron such that they have the same spin before adding an electron to the same orbital but with opposite spin (see the first image).
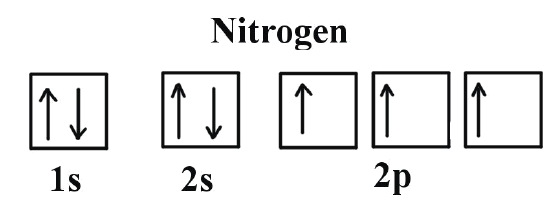
Comparatively, Pauli Exclusion Principle tells us that electrons can never have the same set of quantum numbers (n, l, ml, ms). It tells us that electrons in the same orbital must have different magnetic spins quantum numbers, and thus tells us how to write the electrons out (see the second image)


Comparatively, Pauli Exclusion Principle tells us that electrons can never have the same set of quantum numbers (n, l, ml, ms). It tells us that electrons in the same orbital must have different magnetic spins quantum numbers, and thus tells us how to write the electrons out (see the second image)

-
Kimberly_martinez2I
- Posts: 104
- Joined: Fri Sep 24, 2021 6:25 am
Re: Hund's Rule vs Pauli Exclusion Principle
Hund's Rule states that the electrons would rather be single in an orbital before they bunk up with another electron. While the Pauli exclusion principal states that no 2 electrons in the same atom can have identical sets of quantum numbers, where they can be similar in all quantum numbers except spin.
-
Michelle Gong
- Posts: 104
- Joined: Fri Sep 24, 2021 7:10 am
Re: Hund's Rule vs Pauli Exclusion Principle
Hund's rule explains why orbitals must be singularly occupied before being doubly occupied - in other words, it is why there will be unpaired electrons in an energy level.
Pauli's exclusion principle states that every atom will have its own set of quantum numbers and it is why no two electrons can have the same two quantum numbers. Electrons within an orbital must spin in different directions.
Pauli's exclusion principle states that every atom will have its own set of quantum numbers and it is why no two electrons can have the same two quantum numbers. Electrons within an orbital must spin in different directions.
-
Brooklyn Burgess 3L
- Posts: 111
- Joined: Fri Sep 24, 2021 5:42 am
Re: Hund's Rule vs Pauli Exclusion Principle
Pauli Exclusion Principle:
-no more than 2e- per orbital (state)
-if 2e- are in same orbital then spin paired
Hund's Rule:
-due to e- repulsion, e- in same subshell occupy different orbitals with parallel spin (lowest energy)
-e- in outer most shells are valence e- and are involved in forming bonds
-we apply these rules to obtain our ground state e- configuration matches experimentally observed e- configuration
-no more than 2e- per orbital (state)
-if 2e- are in same orbital then spin paired
Hund's Rule:
-due to e- repulsion, e- in same subshell occupy different orbitals with parallel spin (lowest energy)
-e- in outer most shells are valence e- and are involved in forming bonds
-we apply these rules to obtain our ground state e- configuration matches experimentally observed e- configuration
-
Caitlin_Chheda_1K
- Posts: 35
- Joined: Mon Jan 09, 2023 8:35 am
Re: Hund's Rule vs Pauli Exclusion Principle
Hi!
Hund's Rule states that every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin. Or, that an electron would rather be unpaired than paired at first.
The Pauli Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers. So, there must be a spin (positive or negative) also associated with each electron.
I have also attached pictures.
I hope that this helps!
Hund's Rule states that every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin. Or, that an electron would rather be unpaired than paired at first.
The Pauli Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers. So, there must be a spin (positive or negative) also associated with each electron.
I have also attached pictures.
I hope that this helps!
Return to “Quantum Numbers and The H-Atom”
Who is online
Users browsing this forum: No registered users and 3 guests

