subshells and orbitals
Moderators: Chem_Mod, Chem_Admin
-
Funmi Baruwa
- Posts: 108
- Joined: Wed Sep 30, 2020 9:50 pm
subshells and orbitals
What is the difference between a subshell and an orbital? I know an orbital has subshells, so maybe it means subshells are parts of orbitals, but if that were the case would that then mean that orbitals could have more than one type of subshell?
-
Faaizah Arshad 1H
- Posts: 174
- Joined: Wed Sep 30, 2020 10:01 pm
- Been upvoted: 2 times
Re: subshells and orbitals
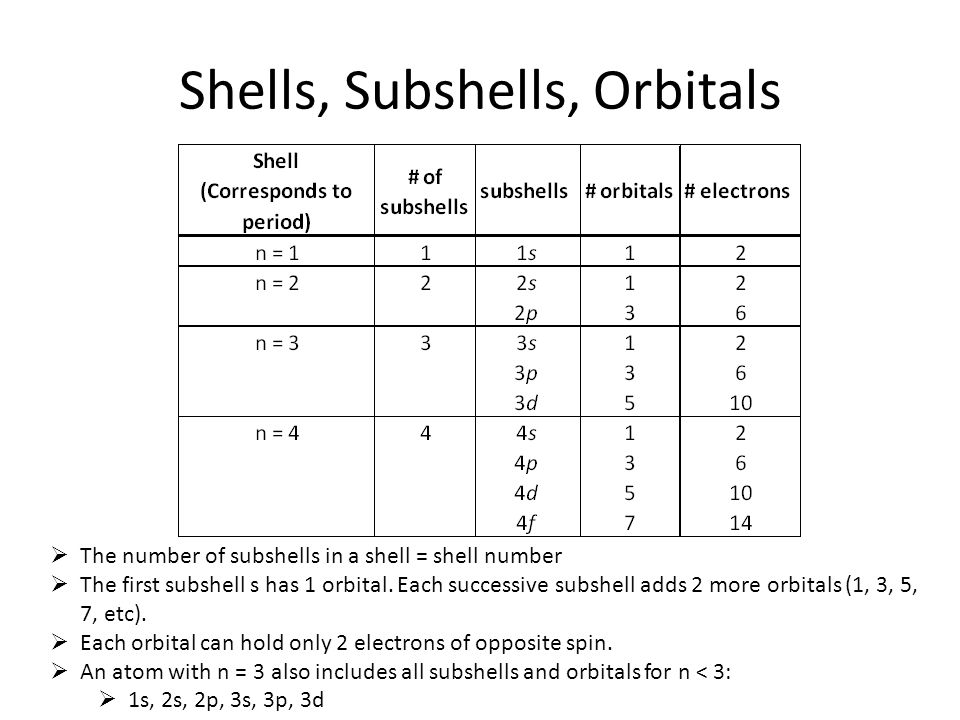
A shell is all orbitals that have the same value of n. For example, the n = 2 shell.
A subshell is all orbitals with the same value of both n and l. For example, the n=2 shell can have l=0,1. A subshell could be 2s, or a subshell could be 2p.
Orbitals have the same value of n, l, ml. For example, the n =2 shell can have l=0,1, and ml = -1,0, +1. If we choose the 2p subshell, then an orbital could be 2px, which is ml = -1.
Hope that helps!
A subshell is all orbitals with the same value of both n and l. For example, the n=2 shell can have l=0,1. A subshell could be 2s, or a subshell could be 2p.
Orbitals have the same value of n, l, ml. For example, the n =2 shell can have l=0,1, and ml = -1,0, +1. If we choose the 2p subshell, then an orbital could be 2px, which is ml = -1.
Hope that helps!
-
Sabina House 2A
- Posts: 102
- Joined: Wed Sep 30, 2020 9:35 pm
- Been upvoted: 4 times
Re: subshells and orbitals
To expand, orbitals are part of subshells, and can hold either the same or less electrons. One example of a subshell would be 2s, which can only hold 2 electrons because it contains one orbital (and each orbital holds 2 electrons). However, the subshell 2p has three orbitals in it so it can hold 6 electrons, which is greater than the amount of electrons an orbital can hold. How I think of it is that subshells are contained within shells and orbitals are contained within subshells.
-
annabelchen2a
- Posts: 108
- Joined: Wed Sep 30, 2020 10:01 pm
-
Funmi Baruwa
- Posts: 108
- Joined: Wed Sep 30, 2020 9:50 pm
-
Chinmayi Mutyala 3H
- Posts: 105
- Joined: Wed Sep 30, 2020 9:50 pm
Re: subshells and orbitals
I think subshell is the combination of the shell and the which orbital it is (which would be represented by l). I'm not sure if this is the best way of explaining it.
-
Eileen Quach Dis 2A
- Posts: 199
- Joined: Wed Sep 30, 2020 9:49 pm
Re: subshells and orbitals
A subshell would be like the s,p,d, and f subshells. The s subshell has 1 orbital, p has 3 orbitals, d has 5 orbitals, and f has 7 orbitals (add 2 each time). Each orbital can "hold" a different number of electrons. For instance, the s subshell can hold 2 electrons in its one orbital and the p subshell can hold 6 electrons in its 3 orbitals. Each subshell can "hold" a number of electrons equal to two times its number of orbitals. Hope that helps clarify things!
-
Earl Garrovillo 2L
- Posts: 101
- Joined: Wed Sep 30, 2020 9:55 pm
Re: subshells and orbitals
I think you have it backward, orbitals are parts of a subshell, not the other way around. The order goes shell>subshell>orbital. Within a given subshell (s,p,d,f), there is a certain number of orbitals that can each contain 2 electrons.
-
Vanshika Bhushan 1A
- Posts: 100
- Joined: Wed Sep 30, 2020 9:33 pm
Re: subshells and orbitals
A shell contains one or more subshells. A subshell contains one or more orbitals. An orbital can contain up to 2 electrons. Shells are assigned a principle quantum number, n (n = 1, 2, 3, ...). Subshells are labeled differently as s, p, d, f. The orbitals are the specific region of space where the electron can be found, and are labeled, for example, px, py and pz.
Return to “Quantum Numbers and The H-Atom”
Who is online
Users browsing this forum: No registered users and 3 guests