Names of different orbitals
Moderators: Chem_Mod, Chem_Admin
-
Jordan_OBrien_2k
- Posts: 53
- Joined: Wed Sep 30, 2020 9:43 pm
Names of different orbitals
I know that the p subshell has specific names for its orbitals: px, py, and pz. But do the 5 orbitals in d and the 7 orbitals in f also have a similar way to differentiate which specific orbital you are talking about?
-
Ashlen Bullock 1H
- Posts: 122
- Joined: Wed Sep 30, 2020 9:58 pm
- Been upvoted: 1 time
Re: Names of different orbitals
The f and d orbitals have a similar way to differentiate, but I do not think you have to memorize them.
-
Gabby Magat 3F
- Posts: 114
- Joined: Wed Sep 30, 2020 10:04 pm
Re: Names of different orbitals
Yeah, I'm pretty sure! Though I don't think we would be expected to name them exactly (I wasn't sure so I looked it up and added a picture here), but I could see a question asking us for the p-orbital since we know 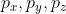 . For d and f-subshells, we would probably have to know that n=3, l=2 would only allow an
. For d and f-subshells, we would probably have to know that n=3, l=2 would only allow an  .
.
Some of the images that pop up with the d and f-subshells refer to the orbitals just by number (like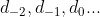 for example. The image I attached is a bit complex, so I don't think we would be expected to know those exact names, just the principle that ml = -1 refers to a different orbital than ml = 1, etc.
for example. The image I attached is a bit complex, so I don't think we would be expected to know those exact names, just the principle that ml = -1 refers to a different orbital than ml = 1, etc.
Some of the images that pop up with the d and f-subshells refer to the orbitals just by number (like
-
ShinwooKim_3E
- Posts: 100
- Joined: Wed Sep 30, 2020 10:00 pm
Re: Names of different orbitals
I don't think we have to know the names and shapes past the d orbitals.
-
Lizbeth Garcia 1F
- Posts: 102
- Joined: Wed Sep 30, 2020 9:39 pm
Re: Names of different orbitals
I think they do have specific names. If you want to know how they look, the book has pictures of them, but we do not need to actually learn their names.
-
Kayla Booker 1F
- Posts: 99
- Joined: Wed Sep 30, 2020 9:43 pm
Re: Names of different orbitals
They do, but I don't think we have to have them memorized, at least not for 14A
-
Jordan_OBrien_2k
- Posts: 53
- Joined: Wed Sep 30, 2020 9:43 pm
Re: Names of different orbitals
Gabby Magat 1K wrote:Yeah, I'm pretty sure! Though I don't think we would be expected to name them exactly (I wasn't sure so I looked it up and added a picture here), but I could see a question asking us for the p-orbital since we know. For d and f-subshells, we would probably have to know that n=3, l=2 would only allow an
.
Some of the images that pop up with the d and f-subshells refer to the orbitals just by number (likefor example. The image I attached is a bit complex, so I don't think we would be expected to know those exact names, just the principle that ml = -1 refers to a different orbital than ml = 1, etc.
Thank you so much! This was really helpful
-
Karina Grover 1A
- Posts: 101
- Joined: Wed Sep 30, 2020 9:34 pm
- Been upvoted: 1 time
Re: Names of different orbitals
Yes, the d and f orbitals have different (specific) configurations just as the p orbital does. However, I do not think it is necessary to memorize the specifics of these orbitals, as they are far more complicated and not entirely of focus in this class.
Return to “Quantum Numbers and The H-Atom”
Who is online
Users browsing this forum: No registered users and 4 guests

