21. How many electrons in an atom could have these sets of quantum numbers?
n = 7, l = 2, m_l = -1
I was a bit confused on this question because I knew that l = 2 meant a d orbital, but there exists no 7d level?
Thanks in advance!
HW2, #21
Moderators: Chem_Mod, Chem_Admin
-
BriannaDis2C
- Posts: 103
- Joined: Fri Sep 24, 2021 6:35 am
Re: HW2, #21
In this circumstance, there would be 2 electrons because l=2 is the third sub shell (7d sub shell) and ml=-1 would be representing the 7d sub shell.
-
KyleNagasawaDisc3C_Chem 14B2022W_
- Posts: 107
- Joined: Fri Sep 24, 2021 5:14 am
- Been upvoted: 6 times
Re: HW2, #21
Hi Jacqueline,
You are absolutely correct in stating that there are two electrons that can maintain the characterizations described by the quantum number. For the sake of the question, I believe that n=7 represents a theoretical principle quantum number. In the same way that g, h, and I subshells are theorized to exist, despite not being present in the ground-state for the known elements, n=7 is moreso a hypothetical example.
You are absolutely correct in stating that there are two electrons that can maintain the characterizations described by the quantum number. For the sake of the question, I believe that n=7 represents a theoretical principle quantum number. In the same way that g, h, and I subshells are theorized to exist, despite not being present in the ground-state for the known elements, n=7 is moreso a hypothetical example.
-
Andrew Nguyen 1E
- Posts: 101
- Joined: Fri Sep 24, 2021 6:34 am
- Been upvoted: 1 time
Re: HW2, #21
Hello,
For this question, we can imagine the 7d subshell, despite it not being on the periodic table, since it is scientifically possible to exist. With n=7, l can equal 2, thus forming the 7d subshell. And since d-subshells have 5 orbitals and each orbital can hold 2 electrons, he 7d subshell will hold up to 10 electrons.
This question is asking if the listed quantum numbers can physically exist. So, it's asking to see if you understand the rules to quantum numbers (l cannot be higher than n,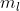 is the negative to positive range of l, and that no two electrons can have the same 4 quantum numbers).
is the negative to positive range of l, and that no two electrons can have the same 4 quantum numbers).
For this question, we can imagine the 7d subshell, despite it not being on the periodic table, since it is scientifically possible to exist. With n=7, l can equal 2, thus forming the 7d subshell. And since d-subshells have 5 orbitals and each orbital can hold 2 electrons, he 7d subshell will hold up to 10 electrons.
This question is asking if the listed quantum numbers can physically exist. So, it's asking to see if you understand the rules to quantum numbers (l cannot be higher than n,
Return to “Quantum Numbers and The H-Atom”
Who is online
Users browsing this forum: No registered users and 8 guests

