Page 1 of 1
Electron Configurations
Posted: Tue Oct 22, 2019 10:41 pm
by salvadorramos3k
Hi can somebody please explain to me how you know the order when writing an electron configuration? For example, 1s, 2s, 2p....Is there a trick to remember the order? Also, how do you know how many electrons will fit in that orbital? Let me know if I have to clarify any parts of the question. Thank you :)
Re: Electron Configurations
Posted: Tue Oct 22, 2019 11:14 pm
by Sophia Shaka 3L
The 'trick' would probably just be having a copy of the periodic table handy, as if you know where the s- and p- blocks are, it is easy to see which subshells fill up before the others. Also, since the order is always the same, a bit of practice will make the order seem more intuitive, but you can always go back to the periodic table and count it out using the rows and columns. As for orbitals, there are ALWAYS only 2 electrons per orbital. This is part of the Pauli Exclusion Principle, because in each orbital, there is one electron with "spin up" and one with "spin down". The s subshell has 1 orbital, the p subshell has 3 orbitals (x, y, z), etc.
Re: Electron Configurations
Posted: Wed Oct 23, 2019 12:10 am
by Brooke Yasuda 2J
Yeah, look at the periodic table. But also, you can just know certain facts like when n = 1, there is only the s subshell. For n = 2, there is only the s and p subshell. for n = 3, there are s, p, and d subshells, and so on. And when writing electron configurations, you always write in the order of s, p, d, f
Re: Electron Configurations
Posted: Wed Oct 23, 2019 12:35 am
by A Raab 1K
To add on the information already provided, the order goes s, p, d, f. For s, there's a maximum of 2 electrons; for p there's 6; for d there's 10 and for f there's 14. Hope that helps when you write it out.
Re: Electron Configurations
Posted: Wed Oct 23, 2019 12:43 am
by Kavya Immadisetty 2B
The easiest way is to use the periodic table but you can also use this chat:
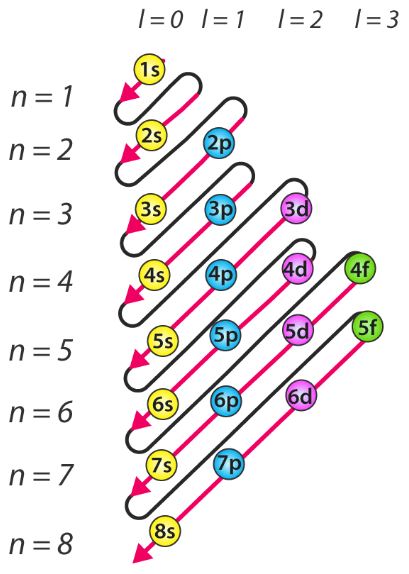
Re: Electron Configurations
Posted: Wed Oct 23, 2019 5:27 pm
by Sara Richmond 2K
Hey the order of electron shell energy is difficult to understand. The only way that I can remember the order of the energy is with this chart. Follow the arrows from bottom to top and then return to the bottom of the next arrow.
Re: Electron Configurations
Posted: Wed Oct 23, 2019 8:16 pm
by Jorge Ramirez_4H
What does he mean when he writes x, y, and z during electron configuration? Is that necessary?
Re: Electron Configurations
Posted: Wed Oct 23, 2019 11:33 pm
by Ryan Chang 1C
Jorge Ramirez_4H wrote:What does he mean when he writes x, y, and z during electron configuration? Is that necessary?
x, y, and z represent the different orbital orientations. Some orbitals may be lined up along x axis, others along the y axis, and others along the z axis. The reason why you write x, y, or z is to specify the orbital orientation.
Re: Electron Configurations
Posted: Thu Oct 24, 2019 9:29 am
by Caroline Zepecki
It's important that you memorize the different groups while looking at the periodic table. The lefthand side has the s groups, the middle the d groups, and the right side the p groups. Then, the constant that determines which orbital it is corresponds to the period # (for s and p groups) and period value -1 for the d group.
Re: Electron Configurations
Posted: Thu Oct 24, 2019 10:27 am
by Jasmine Fendi 1D
Hi, can someone please further explain the exception for the electron configuration for 3d and 4s? especially for the elements Cr and Cu?
Thank you!
Re: Electron Configurations
Posted: Thu Oct 24, 2019 10:31 am
by emma brinton_3B
Hi! i would just recommend copying this chart whenever you come across a question asking for the electron configuration of an element
Re: Electron Configurations
Posted: Thu Oct 24, 2019 11:20 am
by Nathan Nakaguchi 1G
For me, I don't memorize the order I just have memorized the exceptions like when you get to Cr the D shell can have 10 electrons and Cu's 4s shell can only hold 1 electron. The main thing I try to do when finding electron configuration is to build up from the shells (counting the electrons) and to not get confused I just remember that the energy levels must go in increasing order which is why 3d shell comes before the 4s shell (n=3 for d and n=4 for s).
Re: Electron Configurations
Posted: Thu Nov 07, 2019 2:21 pm
by Jorge Ramirez_4H
Is there an easy way to memorize the quantum numbers?
Re: Electron Configurations
Posted: Sun Nov 10, 2019 3:27 pm
by Bradley Whitworth 4B
The way lavelle has us writing it is in order of which electrons will ionize last to the ones that'll ionize first which basically means you write the configuration in order of the principle quantum number which for this class means you need to know that 3d^10 would be listed before 4s^2 in an electron configuration.
