I was under the impression that the in the filling order for orbitals, 5s comes before 4d. Therefore, I would assume the electron configuration of Silver to be:
Ag: [Kr] 4d^9, 5s^2
However, the solution manual has it as [Kr] 4d^10, 5s^1.
Am I wrong and the 5s actually fills before the 4d shell or is Silver's configuration abnormal for some reason? Could someone please explain this to me?
Electron Configuration of Silver
Moderators: Chem_Mod, Chem_Admin
-
Bailey Giovanoli 1L
- Posts: 115
- Joined: Wed Sep 30, 2020 9:50 pm
-
Mohamed Mido
- Posts: 140
- Joined: Wed Sep 30, 2020 9:33 pm
Re: Electron Configuration of Silver
The d orbital is filled before the s orbital because the electron is going to fill up the orbital with the lower energy and one that is more stable. It won't always be the s orbital that is lower energy and more stable. Sometimes the d orbital must be filled completely and then the s orbital starts to get filled up.
-
Samantha Low 3D
- Posts: 117
- Joined: Wed Sep 30, 2020 10:04 pm
Re: Electron Configuration of Silver
Thank you for posting! I always get confused on how the energy orbitals fill up, so I started using this chart. Hope this helps!
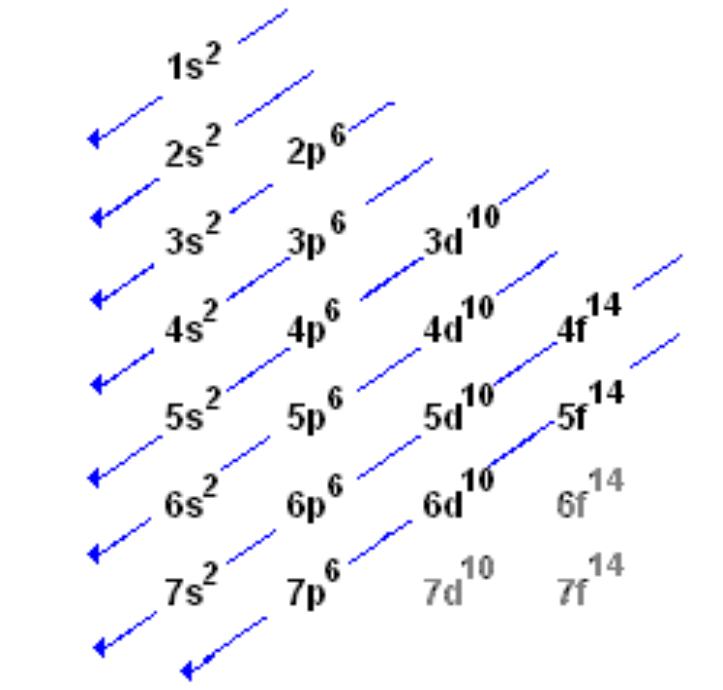

-
Jordan Tatang 3L
- Posts: 125
- Joined: Wed Sep 30, 2020 9:31 pm
- Been upvoted: 3 times
Re: Electron Configuration of Silver
Hi! So you're correct in that you the 5s orbital would get filled prior to the 4d orbitals. However, silver's electron configuration is an exception similar to copper. The 4d orbital is much more stable with 10 electrons instead of 9 so it takes one of the electrons from the 5s orbital.
Hopefully that helps answer your question.
Hopefully that helps answer your question.
Return to “Electron Configurations for Multi-Electron Atoms”
Who is online
Users browsing this forum: No registered users and 10 guests

