Since it will need 2 more electrons to complete the p orbital, will the element S have a negative 2 charge,
Exercise 2A.15 a
Moderators: Chem_Mod, Chem_Admin
-
SamanyaCMuyunga1K
- Posts: 11
- Joined: Wed Sep 30, 2020 10:02 pm
Exercise 2A.15 a
Write the most likely charge for the ions formed by each of the following elements: (a) S .
Since it will need 2 more electrons to complete the p orbital, will the element S have a negative 2 charge,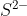 ?
?
Since it will need 2 more electrons to complete the p orbital, will the element S have a negative 2 charge,
-
Victor Li 2A
- Posts: 106
- Joined: Wed Sep 30, 2020 10:08 pm
- Been upvoted: 2 times
Re: Exercise 2A.15 a
You are correct.
S would want to have a full octet valence shell. It already has 6 ve- so it will want to gain 2 e- (so 2- charge) to have a full octet.
S would want to have a full octet valence shell. It already has 6 ve- so it will want to gain 2 e- (so 2- charge) to have a full octet.
-
ellenulitsky Dis 1I
- Posts: 102
- Joined: Wed Sep 30, 2020 9:38 pm
- Been upvoted: 1 time
Re: Exercise 2A.15 a
I also agree that that is correct! Also, I like to look at the periodic table and know that for that column, the charge is 2-, because then it gets to that full octet! Hope that helps.
-
JaesalSoma1E
- Posts: 101
- Joined: Wed Sep 30, 2020 10:02 pm
- Been upvoted: 3 times
Re: Exercise 2A.15 a
Yes, the sulfur will have a -2 charge on it. If you look at the group sulfur is in, you'll notice that all those elements also from -2 charges when they complete their octets.
-
John_Tran_3J
- Posts: 108
- Joined: Wed Sep 30, 2020 9:58 pm
Re: Exercise 2A.15 a
Correct! S is near a full valence shell, so it will be greedy and take the other valence electrons.
Return to “Electron Configurations for Multi-Electron Atoms”
Who is online
Users browsing this forum: No registered users and 5 guests

