Electron Configuration
Moderators: Chem_Mod, Chem_Admin
-
Kimberly_Gutierrez_3B
- Posts: 50
- Joined: Fri Sep 24, 2021 6:42 am
-
Maxwell Yao
- Posts: 102
- Joined: Fri Sep 24, 2021 5:38 am
Re: Electron Configuration
The first step of writing electron configurations is figuring out what element we are working with. If you know what element it is, you can use your periodic table to see how many electrons it should have (should be the same as its atomic number).
After that we can start writing out our electron configurations starting from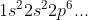
In the electron configurations, the numbers represent the energy level (in other words, n). The letters represent the type of orbitals (s, p, d, f...) and the exponent represents how many electrons are currently filling that specific subshell (the sum of all the exponents should be equal to the number of electrons the atom has).
For example, if we were asked to write the electron configuration for Na (sodium) we know that sodium has 11 electrons and that its valence electron is in the 3rd shell and in the 3s orbital (we know this because of the way the periodic table is organized). Then we start writing it out as such: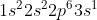
After that we can start writing out our electron configurations starting from
In the electron configurations, the numbers represent the energy level (in other words, n). The letters represent the type of orbitals (s, p, d, f...) and the exponent represents how many electrons are currently filling that specific subshell (the sum of all the exponents should be equal to the number of electrons the atom has).
For example, if we were asked to write the electron configuration for Na (sodium) we know that sodium has 11 electrons and that its valence electron is in the 3rd shell and in the 3s orbital (we know this because of the way the periodic table is organized). Then we start writing it out as such:
-
August_Blatney_1A
- Posts: 52
- Joined: Fri Sep 24, 2021 6:23 am
Re: Electron Configuration
When writing electron figurations there is also a shorthand notation for atoms with more electrons then Ne on the periodic table.
For example when we write the electron configuration for an atom like Al we can write
[Ne] 3s^2 3p^1
This means that Al would be written the same way as Ne to start and then the 3s^2 and 3p^1 would be added to that to make up its electron configuration
The same can be done for an atom like Sc which can be written as
[Ar] 3d^1 4s^2
For example when we write the electron configuration for an atom like Al we can write
[Ne] 3s^2 3p^1
This means that Al would be written the same way as Ne to start and then the 3s^2 and 3p^1 would be added to that to make up its electron configuration
The same can be done for an atom like Sc which can be written as
[Ar] 3d^1 4s^2
-
Cecilia Lei 3K
- Posts: 107
- Joined: Fri Sep 24, 2021 5:12 am
Re: Electron Configuration
To write electron configurations, I think the best way is to see whether it can be write in a simplified form or not, because it will be easier for you to construct a configuration with the help of inert gases like helium. The second step is to see where is the element locate in the periodic table and see which block it is in, whether it's in the block of s, p, or d, or f.
Note: first row d-block element is the most stable when it is half-filled or fully filled.
Note: first row d-block element is the most stable when it is half-filled or fully filled.
Return to “Electron Configurations for Multi-Electron Atoms”
Who is online
Users browsing this forum: No registered users and 5 guests

