Exceptions
Moderators: Chem_Mod, Chem_Admin
Re: Exceptions
Unfortunately, I think these are really ones that we have to memorize. But it helps me that they are similar letters that have exceptions (ie the elements that have exceptions are Cr and Cu).
-
Ethan Hung 2A
- Posts: 103
- Joined: Fri Sep 24, 2021 6:02 am
Re: Exceptions
For the d-orbital exceptions, you're mostly looking at atoms with a ground state of nd4 or nd9 elements, where n = 3, 4, ...
This basically means the exceptions are group 6 and 11 on the periodic table, for most intents and purposes. For period 11, you can think of them as the valuable metals (copper, silver, gold), and for group 6 you can try making a mnemonic -- though, realistically you'll just use your periodic table to check whenever you see a question on the midterm/final that involves a transition metal.
This basically means the exceptions are group 6 and 11 on the periodic table, for most intents and purposes. For period 11, you can think of them as the valuable metals (copper, silver, gold), and for group 6 you can try making a mnemonic -- though, realistically you'll just use your periodic table to check whenever you see a question on the midterm/final that involves a transition metal.
-
Alison King 3L
- Posts: 113
- Joined: Fri Sep 24, 2021 5:33 am
- Been upvoted: 3 times
Re: Exceptions
The elements with d4 or d9 change to fill the d orbital d5 or d10 and remove one from the s orbital. I think we only need to know Chromium and Copper for the exam
-
Brian Chau 1D
- Posts: 50
- Joined: Fri Sep 24, 2021 6:46 am
Re: Exceptions
The way I think about it is that both the s- and d- orbital are half full for these exceptions, making the electron configuration more stable. For example, chromium would have [Ar]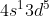 . Both the s-orbital (which can hold 2 electrons) and d-orbital (which can hold 10 electrons) are half full instead of just the s-orbital being full.
. Both the s-orbital (which can hold 2 electrons) and d-orbital (which can hold 10 electrons) are half full instead of just the s-orbital being full.
-
Cindy Vo 3E
- Posts: 100
- Joined: Fri Sep 24, 2021 6:52 am
Re: Exceptions
In general, I memorized that the Cr and Cu groups have exceptions for the electron configurations because both have a C in them. But, in the sense of writing it, I just think that if s is full and d is one away from being half full (d4) or full (d10) when I'm looking at the periodic table, s will give one to d so that d is half full or full.
Return to “Electron Configurations for Multi-Electron Atoms”
Who is online
Users browsing this forum: No registered users and 4 guests

