Determining ml quantum number [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
Determining ml quantum number
Hey, so I understand stand how we can determine all of the quantum numbers except for the third one : ml. I understand what the range is and how it is related to the previous numbers, but how do we find the exact number (ex -1, 0, 1, 2.... etc) for a specific electron? Is it just in order?
-
Sadhana Jeyakumar 2J
- Posts: 144
- Joined: Fri Sep 24, 2021 5:25 am
- Been upvoted: 1 time
Re: Determining ml quantum number
To determine the specific 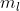 quantum number, I place the electrons in order to see where the last electron is placed.
quantum number, I place the electrons in order to see where the last electron is placed.
For example, if I am trying to find the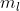 quantum number for the fourth electron in
quantum number for the fourth electron in 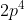 , I know that n = 2 and l = 1. As l = 1, possible values for
, I know that n = 2 and l = 1. As l = 1, possible values for 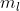 include -1, 0, and 1.
include -1, 0, and 1.
Because there are 4 electrons in the p orbital, the fourth electron must lie in the orbital (applying Hund's rule). Therefore,
orbital (applying Hund's rule). Therefore, 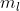 = -1
= -1
I hope that helps!
For example, if I am trying to find the
Because there are 4 electrons in the p orbital, the fourth electron must lie in the
I hope that helps!
-
Meghan Spoeri 2D
- Posts: 101
- Joined: Fri Sep 24, 2021 6:01 am
- Been upvoted: 5 times
Re: Determining ml quantum number
Hi!
ml corresponds to the orientation of the electron, and it labels different orbitals of a subshell. Each ml value corresponds to a specific l, for instance, l=1 can have a ml value of -1, 0, or 1. There are 3 different ml values for l=1 because l=1 corresponds to the p orbital, and there are 3 orbitals within the p subshell, px, py, and pz. -1,0, and 1 each correspond to a different orbital px, py, and pz.
ml corresponds to the orientation of the electron, and it labels different orbitals of a subshell. Each ml value corresponds to a specific l, for instance, l=1 can have a ml value of -1, 0, or 1. There are 3 different ml values for l=1 because l=1 corresponds to the p orbital, and there are 3 orbitals within the p subshell, px, py, and pz. -1,0, and 1 each correspond to a different orbital px, py, and pz.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Determining ml quantum number
Ml will always range from (-l)-(+l). So for example, if you have a quantum number l (angular momentum quantum number) value of 2, all the possible ml values are; -2,-1,0,1,2. So there are multiple orbitals that can have the same n and l combination but different ml (magnetic quantum number).
-
Martha Avila 1I
- Posts: 100
- Joined: Fri Sep 24, 2021 6:21 am
- Been upvoted: 1 time
Re: Determining ml quantum number [ENDORSED]
Hello. So when determining the ml quantum number you will always have a range of possible values unless directly specified. For example if you know that n=3 you know that l = 2 and this entails that all of your possible ml values are -2,-1,0,1,2. You can be told to give all possible values of ml and in this case you would be done but if you are told to find the exact value you must keep in mind the number of orbitals as well as electrons.
-
Michelle Li 2B
- Posts: 109
- Joined: Fri Sep 24, 2021 5:11 am
- Been upvoted: 1 time
Re: Determining ml quantum number
Hi! If I understand correctly, you're asking how to determine which ml number corresponds to each orientation/electron in the electron configuration? So like which values of ml = -1, 0, +1 would be px, py, or pz? I was also confused about this and asked in a review session today, and the TA said that we probably won't have to assign ml values to a certain orientation x, y, or z, since it's random. That's what I learned from the session, but anyone feel free to correct me if Dr. Lavelle or another TA said something different!
Return to “Electron Configurations for Multi-Electron Atoms”
Who is online
Users browsing this forum: No registered users and 7 guests

