m(subscript L)
Moderators: Chem_Mod, Chem_Admin
-
Ellen Tsai 3D
- Posts: 55
- Joined: Fri Sep 24, 2021 5:55 am
m(subscript L)
I'm just a little confused at how one can tell what m(l) is. Like I understand that if you're given 4p2 n = 4, l = 1 and m(l) can be anything from -1, 0 , 1 but how do you know exactly. Thank you!
-
Anna Hilger 3H
- Posts: 98
- Joined: Fri Sep 24, 2021 7:09 am
- Been upvoted: 1 time
Re: m(subscript L)
Hi,
It depends on the number of valence electrons. For example, 4p^2, there are 2 valence electrons. So, the first electron goes into either -1 or +1 and then the second goes into 0. I don't know if this makes sense, but it is how I understand it.
Hope this helps :)
It depends on the number of valence electrons. For example, 4p^2, there are 2 valence electrons. So, the first electron goes into either -1 or +1 and then the second goes into 0. I don't know if this makes sense, but it is how I understand it.
Hope this helps :)
-
Ellen Tsai 3D
- Posts: 55
- Joined: Fri Sep 24, 2021 5:55 am
Re: m(subscript L)
Anna Hilger 2G wrote:Hi,
It depends on the number of valence electrons. For example, 4p^2, there are 2 valence electrons. So, the first electron goes into either -1 or +1 and then the second goes into 0. I don't know if this makes sense, but it is how I understand it.
Hope this helps :)
wait so how do uk whether it goes into -1 or +1 first...
-
bree_wray_14a
- Posts: 50
- Joined: Fri Sep 24, 2021 6:33 am
Re: m(subscript L)
For the s orbital, the maximum amount of orbitals is 1. For the p orbital, the max orbital number is 3. for d orbital it is 5. and for f orbital it is 7. each ml value begins with zero and move outward in both directions from there.
I hope that makes sense!!!
I hope that makes sense!!!
-
Anna Hilger 3H
- Posts: 98
- Joined: Fri Sep 24, 2021 7:09 am
- Been upvoted: 1 time
Re: m(subscript L)
Ellen Tsai 3D wrote:Anna Hilger 2G wrote:Hi,
It depends on the number of valence electrons. For example, 4p^2, there are 2 valence electrons. So, the first electron goes into either -1 or +1 and then the second goes into 0. I don't know if this makes sense, but it is how I understand it.
Hope this helps :)
wait so how do uk whether it goes into -1 or +1 first...
I don't know. My TA said either it would be in -1 or +1, so I guess you need complicated math to find out.
-
Sanjana Sivakumar 2E
- Posts: 125
- Joined: Fri Sep 24, 2021 7:30 am
- Been upvoted: 2 times
Re: m(subscript L)
Ellen Tsai 3D wrote:Anna Hilger 2G wrote:Hi,
It depends on the number of valence electrons. For example, 4p^2, there are 2 valence electrons. So, the first electron goes into either -1 or +1 and then the second goes into 0. I don't know if this makes sense, but it is how I understand it.
Hope this helps :)
wait so how do uk whether it goes into -1 or +1 first...
Hello!
From what I understand, the ml represents the orientation of the orbital. And since orientation is in 3D, it's non-directional. I can turn the sphere any way and relabel the axises, but the sphere itself won't change. So, I think it wouldn't matter which goes first. And I think that's as much as we need to know. I'm not sure if I'm explaining this right, but this is what I think it is.
Hope this helps!
-
Kathryn Heinemeier 3H
- Posts: 104
- Joined: Fri Sep 24, 2021 5:09 am
Re: m(subscript L)
it depends on what orbital it is because a s orbital will be 0 while a p orbital will be -1,0,1
-
Jane Wang 1E
- Posts: 115
- Joined: Fri Sep 24, 2021 6:36 am
Re: m(subscript L)
Hi! Basically m(l) is the magnetic quantum number, and it represents the orbital, for example, in p subshell, it has Px, Py and Pz three orbitals. Plus, the value of m(l) are from -l to +l, including 0.
Hope this helps!
Hope this helps!
-
Mona Reddy Kurra 1J
- Posts: 100
- Joined: Fri Sep 24, 2021 5:16 am
- Been upvoted: 1 time
Re: m(subscript L)
Hi! Similar to what the previous posts said, 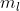 refers to the specific orbital's orientation. My TA said that because of this, the values are arbitrarily assigned. Although you can determine what the possible values of
refers to the specific orbital's orientation. My TA said that because of this, the values are arbitrarily assigned. Although you can determine what the possible values of 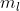 are based on the subshell information, Hund's rule, and the number of electrons that the atom has, the actual assignment of the value to each electron doesn't really matter since the orientation is based on our own perspective of the 3D space. I hope that helps!
are based on the subshell information, Hund's rule, and the number of electrons that the atom has, the actual assignment of the value to each electron doesn't really matter since the orientation is based on our own perspective of the 3D space. I hope that helps!
-
Elsie_Lin_2K
- Posts: 103
- Joined: Fri Sep 24, 2021 5:08 am
Re: m(subscript L)
m(l) represents the orientation of the orbitals so in order to determine the value of m(l) you would have to look at the orbital by looking at the value of l. You can calculate the values of m(l) using l, l-1,...-l. The different orbitals have a maximum number of orientations. For example, there are 3 in the p orbital and 5 in the d orbital.
-
Isabelle Kim 3E
- Posts: 131
- Joined: Fri Sep 24, 2021 6:17 am
- Been upvoted: 1 time
Re: m(subscript L)
Kind of to reiterate what everybody else has been saying, you've got the right idea for sure but to further clarify - this quantum # (ml) describes the possible coordination of the electrons of a particular atom
So using the l value, we may approximate the possible electron configurations within that given l value, which is providing us the shape of orbital (s, p, d, f).
So using the l value, we may approximate the possible electron configurations within that given l value, which is providing us the shape of orbital (s, p, d, f).
Re: m(subscript L)
The ml value is based on the l value. It will range from -l to +l. So the s subshell has a ml value of 0, the p can range from -1, 0, 1, the d could be -2, -1, 0, 1, 2, etc.
Return to “Electron Configurations for Multi-Electron Atoms”
Who is online
Users browsing this forum: No registered users and 4 guests

