When to move electrons from the s orbital to d orbital
Moderators: Chem_Mod, Chem_Admin
-
Jonathan Zuo 2J
- Posts: 35
- Joined: Fri Sep 25, 2015 3:00 am
When to move electrons from the s orbital to d orbital
I remember learning that a half filled or completely filled d orbital is more stable and preferred than a d orbital that is not. For example the electron configuration of Nickel would be [Ar]:3d10 instead of [Ar]3d84s2 because the two electrons in the 4s orbital would go to fill the d orbital. Would we do the same for the electron configuration of V ,[Ar]:3d5, or would it be [Ar]3d34s2? Because on a homework problem the correct answer was [Ar]3d34s2. I guess my question is how do I know when to move electrons from the s orbital to the d orbital.
-
Nitin Joseph
- Posts: 21
- Joined: Fri Sep 25, 2015 3:00 am
Re: When to move electrons from the s orbital to d orbital
The electron config of Nickel would not be 
It's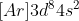
Only one electron can be moved from the s orbital to the d orbital when fulfilling the half-filled subtle rule.
This is why the electron config of Copper is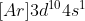
and not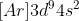
Similarly, the configuration of Vanadium is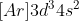 as you cannot move two electrons from the s shell into the d shell.
as you cannot move two electrons from the s shell into the d shell.
However, the next element in the periodic table, Chromium would have a config of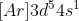 as one electron has been moved to the d subshell.
as one electron has been moved to the d subshell.
So basically, when the half-filled d subshell can be achieved by the movement of one electron, an electron should be moved from the s to the d subshell.
Hope this answers your question.
It's
Only one electron can be moved from the s orbital to the d orbital when fulfilling the half-filled subtle rule.
This is why the electron config of Copper is
and not
Similarly, the configuration of Vanadium is
However, the next element in the periodic table, Chromium would have a config of
So basically, when the half-filled d subshell can be achieved by the movement of one electron, an electron should be moved from the s to the d subshell.
Hope this answers your question.
Return to “Electron Configurations for Multi-Electron Atoms”
Who is online
Users browsing this forum: No registered users and 3 guests

