When converting from grams to moles of say,
Molar Mass
Moderators: Chem_Mod, Chem_Admin
-
Bree Perkins 1E
- Posts: 34
- Joined: Fri Apr 06, 2018 11:04 am
Molar Mass
Hi,
When converting from grams to moles of say,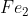 or
or 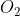 would the grams per mole value be 16 (1 oxygen), or 32 (2 oxygen)?
would the grams per mole value be 16 (1 oxygen), or 32 (2 oxygen)?
When converting from grams to moles of say,
-
Heung Ching Chia 1E
- Posts: 25
- Joined: Mon Apr 09, 2018 1:38 pm
Re: Molar Mass
The grams per mole value (i assume you mean molar mass) would be 32gmol^-1 if you're converting from grams to moles for O2.
-
Miya Lopez 1I
- Posts: 62
- Joined: Tue Nov 14, 2017 3:00 am
- Been upvoted: 1 time
Re: Molar Mass
When finding the molar mass of Fe2, you would look on the periodic table to see the molar mass of just Fe which is 55.845, and multiply it by 2 to get 111.69 g.mol-1 of Fe2.
Re: Molar Mass
It's always the molar mass multiplied by the subscript attached to that element and/or compound. for example (PO2)2 would require you to find the molar mass of PO2 and then multiply it by two because in whatever molecule there are two PO2 molecules bound.
-
Nina Do 4L
- Posts: 61
- Joined: Fri Sep 28, 2018 12:27 am
Re: Molar Mass
You would look at the element first and then see its atomic mass on the periodic table. Ex: O=~16g/mol. And since you're asking about O2, we'd multiply that by 2 because we have two of them so it would be 32g/mol.
-
Archana Biju 1G
- Posts: 29
- Joined: Fri Sep 28, 2018 12:18 am
Re: Molar Mass
Since you are converting O2 and not just oxygen, you would have to multiply the atomic mass of oxygen (15.99) by two to get the molar was for O2. The same goes for F2 too.
Return to “Accuracy, Precision, Mole, Other Definitions”
Who is online
Users browsing this forum: No registered users and 4 guests

