Why we would add
How to calculate molar mass of a natural sample of lithium, referring to textbook Fundamental E11
Moderators: Chem_Mod, Chem_Admin
-
Christian_Lee_2K
- Posts: 174
- Joined: Fri Sep 29, 2023 12:02 pm
How to calculate molar mass of a natural sample of lithium, referring to textbook Fundamental E11
The nuclear power industry extracts 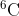 but not
but not 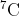 from natural samples of lithium. As a result, the molar mass of commercial samples of lithium is increasing. The current abundances of the two isotopes are 7.42% and 92.58%, respectively, and the masses of their atoms are 9.988*10^-24 and 1.165*10^-23 respectively. (A) what is the current molar mass of a natural sample of lithium?
from natural samples of lithium. As a result, the molar mass of commercial samples of lithium is increasing. The current abundances of the two isotopes are 7.42% and 92.58%, respectively, and the masses of their atoms are 9.988*10^-24 and 1.165*10^-23 respectively. (A) what is the current molar mass of a natural sample of lithium?
Why we would add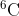 and
and 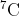 . I thought we would only use
. I thought we would only use 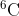 because it is asking for the natural sample and only
because it is asking for the natural sample and only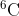 is pulled from a natural sample.
is pulled from a natural sample.
Why we would add
-
Lily Jiang 3K
- Posts: 95
- Joined: Fri Sep 29, 2023 11:36 am
Re: How to calculate molar mass of a natural sample of lithium, referring to textbook Fundamental E11
I think you have misunderstood the question. The problem only mentions the extraction of Li-6 to explain the increasing molar mass of commercial Li. To solve for the molar mass, you still need to use the given abundances for Li-6 and Li-7, not just Li-6.
Molar Mass = (abundance of Li-6 * molar mass of Li-6) + (abundance of Li-7 * molar mass of Li-7)
Molar Mass = (abundance of Li-6 * molar mass of Li-6) + (abundance of Li-7 * molar mass of Li-7)
Return to “Accuracy, Precision, Mole, Other Definitions”
Who is online
Users browsing this forum: No registered users and 4 guests

