Isotopes [ENDORSED]
Moderators: Chem_Mod, Chem_Admin
-
Kristen Laulette 1C
- Posts: 10
- Joined: Fri Apr 06, 2018 11:03 am
Isotopes
I am super rusty on high school chemistry, and don't remember and truly never really got what isotopes are.
What is like a simple way to understand what an isotope is?
What is like a simple way to understand what an isotope is?
-
Lenaschelzig1C
- Posts: 21
- Joined: Fri Apr 06, 2018 11:05 am
Re: Isotopes [ENDORSED]
isotopes have the same number of protons, but different number of neutrons in the nuclei!
-
Corryn Doll
- Posts: 31
- Joined: Fri Apr 06, 2018 11:02 am
Re: Isotopes
Isotopes are forms of the same element which have the same number of protons, but a different number of neutrons, thus giving a varying atomic mass, while the chemical properties of the element remain the same.
Hope this helps.
Hope this helps.
Re: Isotopes
Basically a simple way to think about isotopes are that within the same element there are atoms that have a different number of neutrons but same number of protons. So the atomic mass within different isotopes changes.
(IsoTopes) T for Top meaning the top number (atomic mass changes)
(IsoTopes) T for Top meaning the top number (atomic mass changes)
-
Hongtao Yao
- Posts: 18
- Joined: Fri Apr 06, 2018 11:05 am
Re: Isotopes
Elements exist in different forms. While they all have the same amount of protons in one atom, the number of neutrons in them is different. Every kind of variation of the neutron numbers is an isotope.
-
Brian Chang 2H
- Posts: 65
- Joined: Fri Sep 28, 2018 12:17 am
Re: Isotopes
Most if not all elements exist in different forms. Only Protons and Electrons affect the charge of the atom whereas Neutrons can be added and only affect the weight of the atom. For example, Carbon exists in C-8 to C-15 isotopes where the only thing that changes is the number of Neutrons.
-
deepto_mizan1H
- Posts: 65
- Joined: Fri Sep 28, 2018 12:16 am
Re: Isotopes
Another interesting fact that can help conceptualize the importance of isotopes is that the molar mass of the atoms we have on the Periodic Table are in fact representative of the most commonly (or long-lasting) isotope of the atom we can observe. These will definitely be important as we get to deeper chemistry topics but isotopes can help express the diversity of all the atoms we find, and how they can interact uniquely too.
-
Quinn_Simpson_3D
- Posts: 12
- Joined: Fri Sep 28, 2018 12:26 am
Re: Isotopes
Isotopes are chemical element variants with a different number of neutrons than the original proton amount. This is why the molar mass of elements isn't exactly the same as the protons and neutrons added together. Based on their percentage on Earth, the different element variations are averaged out, causing little changes in mass. For example, Carbon is 12.011 because of the small percentages in Carbon-13 and Carbon-14 on Earth along with the abundant Carbon-12.
-
haleyervin7
- Posts: 61
- Joined: Fri Sep 28, 2018 12:15 am
-
Hailey Boehm 2H
- Posts: 71
- Joined: Fri Sep 28, 2018 12:24 am
Re: Isotopes
From what I remember a common cause for a change in the number of neutrons in an atom is radioactive decay.
-
Liset Rivera 3A
- Posts: 31
- Joined: Fri Sep 28, 2018 12:23 am
Re: Isotopes
This means that the element does not have the same amount of neutrons in the nucleus but does have the same number of protons. This causes the molar mass of an element to vary because the molar mass depends on the sum of the neutrons (which vary) and the protons.
-
Joey Greco 1F
- Posts: 26
- Joined: Fri Sep 28, 2018 12:16 am
Re: Isotopes
Also, isotopes are normally written with a superscript to the top left of the Element, for instance carbon has isotopes from 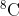 to
to 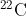
Re: Isotopes
Isotopes are atoms of the same elements but have different mass numbers or different number of protons!
Return to “Accuracy, Precision, Mole, Other Definitions”
Who is online
Users browsing this forum: No registered users and 4 guests

