diatomic elements
Moderators: Chem_Mod, Chem_Admin
-
Leslie Cheng 4B
- Posts: 35
- Joined: Fri Sep 28, 2018 12:29 am
diatomic elements
Hi! Can someone explain to me why diatomic elements form, and how to distinguish between whether an element is existing in its diatomic form or not? For example, if a problem involves a diatomic element like oxygen, do you assume that's in a diatomic form, or does the problem have to state explicitly "oxygen gas"?
-
Erin Kim 2G
- Posts: 75
- Joined: Fri Sep 28, 2018 12:26 am
Re: diatomic elements
All the elements in the acronym HOFBrINCl, (hydrogen, oxygen, fluorine, Bromine, Iodine, and chlorine) are included in the diatomic elements. Any time these elements are listed in a chemical equation they must have the subscript of 2 as you always assume they are diatomic.
-
Elias Omar 1G
- Posts: 52
- Joined: Fri Sep 28, 2018 12:17 am
Re: diatomic elements
The reason these elements are diatomic is because they don't have enough electrons to fill up their valence shells. Thus, they cannot exist as a single atom.
-
Ashish Verma 2I
- Posts: 59
- Joined: Fri Sep 28, 2018 12:28 am
Re: diatomic elements
One cheap mnemonic device to remember these is: Never Have Fear Of Ice CoLd Bread
Never: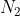 Nitrogen
Nitrogen
Have: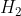 Hydrogen
Hydrogen
Fear: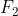 Fluorine
Fluorine
Of: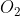 Oxygen
Oxygen
Ice: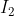 Iodine
Iodine
CoLd: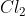 Chlorine
Chlorine
Bread: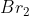 Bromine
Bromine
Never:
Have:
Fear:
Of:
Ice:
CoLd:
Bread:
-
Ester Garcia 1F
- Posts: 29
- Joined: Fri Sep 28, 2018 12:17 am
Re: diatomic elements
My TA taught us another method to remember the diatomic elements. She refers to them as gens and ines. Ex. HydroGEN and fluorINE
-
AJ Manzano 3K
- Posts: 16
- Joined: Fri Sep 28, 2018 12:25 am
Re: diatomic elements
For me, it helps when I picture them on the periodic table because, with the exception of Hydrogen, the other six elements (C, N, O, F, Cl, Br, I) border the top right hand corner of the table next to the column of noble gases.
-
inlovewithchemistry
- Posts: 104
- Joined: Fri Sep 28, 2018 12:19 am
Re: diatomic elements
In high school, we learned to remember them with a phrase pronounced "Br-inkl-hoff," which is Br(bromine), I(iodine), N (nitrogen), Cl(chlorine), H(hydrogen), O(oxygen), and F(fluorine). Helped me a lot back then and now.
-
Aili Ye 4L
- Posts: 58
- Joined: Fri Sep 28, 2018 12:16 am
Re: diatomic elements
Some elements exist as diatomic elements since in their original state, their valence shells are not filled, thus they share electrons between each other and thus fill their shells and achieve a state of lower energy.
-
Andrew Lam 3B
- Posts: 30
- Joined: Fri Sep 28, 2018 12:24 am
Re: diatomic elements
Diatomics exist to share electrons covalently and achieve a lower energy state.
HOFBrINCl is a metonym to remember the diatomics:
H- Hydrogen
O- Oxygen
F- Fluorine
Br- Bromine
I- Iodine
N- Nitrogen
Cl- Chlorine
HOFBrINCl is a metonym to remember the diatomics:
H- Hydrogen
O- Oxygen
F- Fluorine
Br- Bromine
I- Iodine
N- Nitrogen
Cl- Chlorine
-
Karina Jiayu Xu 4E
- Posts: 58
- Joined: Fri Sep 28, 2018 12:29 am
Re: diatomic elements
Does anyone know what order to put the elements in when writing an empirical formula?
-
Rhea Churi 4K
- Posts: 62
- Joined: Fri Sep 28, 2018 12:28 am
Re: diatomic elements
Karina Jiayu Xu 4E wrote:Does anyone know what order to put the elements in when writing an empirical formula?
In an empirical formula, it's typically C, H, and then other elements follow in alphabetical order.
Re: diatomic elements
Diatomic elements only need to exist in pairs of atoms when they are standing alone. For instance, in OH-, you wouldn't need two hydrogen atoms because the hydrogen is not free standing. However, if you have hydrogen gas, you would need H2 because it is not attached to a different element.
Re: diatomic elements
Elements are diatomic because their valence shells aren't completely filled by electrons; therefore, electrons are shared a lower energy state is achieved.
Return to “Accuracy, Precision, Mole, Other Definitions”
Who is online
Users browsing this forum: No registered users and 7 guests

