Hi, I just have quick questions about the Roman numerals next to the metals when they bond with oxygen to become oxide, sulfide, etc.
How do you know which roman numeral to put?
Roman Numerials Next to Metals
Moderators: Chem_Mod, Chem_Admin
-
Xinyu Li 1C
- Posts: 101
- Joined: Wed Sep 30, 2020 9:36 pm
Re: Roman Numerials Next to Metals
The roman numerals indicate the charge and oxidation state of transition metal ions. Typically, you want the overall charge to be zero.
Ex: if sulfide have a charge of -2, you'd want the iron ion to have a charge of +2, written as iron (II) or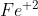
Ex: if sulfide have a charge of -2, you'd want the iron ion to have a charge of +2, written as iron (II) or
-
Silvi_Lybbert_3A
- Posts: 114
- Joined: Wed Sep 30, 2020 9:43 pm
Re: Roman Numerials Next to Metals
Roman numerals appear next to ions that often have different charged forms. For example, iron and copper often have different forms. Iron can have a charge of +2 or +3, while copper can have a charge of +1 or +2. The Roman numeral next to these ions corresponds to their charge (ex/ copper (II) sulfate indicates copper with a charge of +2). Like said above, the overall charge of the molecule should be zero so an ion that can have different charged forms should have the charge that matches the charge (s) of the other atoms in the molecule accordingly. The different forms of the ions have different suffixes as well (ex/ ferric is iron(III) and ferrous is iron(II)), but you do not need to know this.
-
Michelle Nguyen 3F
- Posts: 104
- Joined: Wed Sep 30, 2020 10:03 pm
Re: Roman Numerials Next to Metals
Roman numerals show up most often in transition metals, as depending on what they bond with, they could have different charges. The main approach is when you're given a formula, know the charge of the other atom and then you would know the charge of the transition metal.
For example, if you are given Fe(NO3)3, iron has the possible charges of +2 and +3. First, find the charge of nitrate, which is -1. Since there are 3 nitrate molecules, the total charge of (NO3)3 would be -3. This means that iron should be a charge of +3. You would then right it out as Iron (III) Nitrate.
For example, if you are given Fe(NO3)3, iron has the possible charges of +2 and +3. First, find the charge of nitrate, which is -1. Since there are 3 nitrate molecules, the total charge of (NO3)3 would be -3. This means that iron should be a charge of +3. You would then right it out as Iron (III) Nitrate.
Return to “Accuracy, Precision, Mole, Other Definitions”
Who is online
Users browsing this forum: No registered users and 4 guests

