Avogadro constant
Moderators: Chem_Mod, Chem_Admin
-
Savannah Torella 1L
- Posts: 52
- Joined: Wed Sep 30, 2020 9:40 pm
Avogadro constant
When do you want to use the Avogadro constant? How are you able to tell when it is needed?
-
Taylor K 2D
- Posts: 105
- Joined: Wed Sep 30, 2020 9:35 pm
- Been upvoted: 1 time
Re: Avogadro constant
when converting to moles from number of atoms or vice versa, you would need to use Avogadro's constant.
-
Brandon Pham 1H
- Posts: 82
- Joined: Wed Sep 30, 2020 9:59 pm
- Been upvoted: 1 time
Re: Avogadro constant
Avogadro's number (6x10^23) represents the number of atoms/electrons/molecules in a mole of that substance. You'd want to use it to either convert atoms/electrons/molecules to moles or vice versa
-
Hannah Alltucker 3L
- Posts: 117
- Joined: Wed Sep 30, 2020 9:44 pm
Re: Avogadro constant
Normally you'd want to use it when converting some other unit measurement into atoms/molecules or the other way around (1 mole of anything = 6.022 x 10^23). It's also included in some of the equations we use, but that just means you need to plug it in with the rest of your values. I think the main thing is understanding how it can convert a mole of an element/molecule into how many little parts there are in it.
-
Ke Huang 2G
- Posts: 74
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Avogadro constant
We need to use this constant when we need to know the mass for one atom by dividing the molar mass from periodic table by this constant.
-
Algernon Jackson 2l
- Posts: 52
- Joined: Mon Jan 06, 2020 7:27 am
-
Constance Newell
- Posts: 100
- Joined: Wed Sep 30, 2020 9:38 pm
Re: Avogadro constant
Avogadro converts moles to "things" which is literally anything that isn't already defined: atomps, particles, ect
Re: Avogadro constant
You use this constant to convert almost any quantified value (electrons, molecules, etc. ) into moles and vice versa by either dividing or multiplying by it.
-
Britney Tran IJ
- Posts: 103
- Joined: Fri Aug 30, 2019 12:18 am
Re: Avogadro constant
When a problem asks for the number of photons, atoms, e.t.c you would use Avogadro's number
-
Ivan Chen 2H
- Posts: 104
- Joined: Wed Sep 30, 2020 9:48 pm
Re: Avogadro constant
You can also find the mass of a single atom by dividing its molar mass with Avogadro's constant
-
Bai Rong Lin 2K
- Posts: 101
- Joined: Wed Sep 30, 2020 9:54 pm
Re: Avogadro constant
Savannah Torella 1L wrote:When do you want to use the Avogadro constant? How are you able to tell when it is needed?
It is important to use when converting moles to atoms or vice versa!
-
simona_krasnegor_1C
- Posts: 50
- Joined: Wed Sep 30, 2020 9:35 pm
Re: Avogadro constant
Avogadro's number is used when the question asks for the number of atoms, molecules, or formula units of a specific substance. It is a useful tool for these conversions.
-
Katie Phan 1K
- Posts: 104
- Joined: Wed Sep 30, 2020 9:55 pm
-
Jacob Schwarz-Discussion 3I
- Posts: 111
- Joined: Wed Sep 30, 2020 10:01 pm
Re: Avogadro constant
You use this constant to convert atoms/electrons/molecules into the number of moles, or conversely the number of moles into atoms/electrons/molecules.
Re: Avogadro constant
You use it when converting between mass and mols of a substance. This substance can be an element or molecule.
-
Brian Acevedo 2E
- Posts: 69
- Joined: Wed Sep 30, 2020 9:44 pm
Re: Avogadro constant
The vast majority of the applications of the constant are in problems relating to stoichiometry. The units for the constant (which is used to find number of atoms, as represented by N*subscript A* on the constants and equations sheet) are inverse moles. Therefore it is used when converting between the number of moles and the number of atoms of a particular substance.
-
Dane_Beasley_1E
- Posts: 74
- Joined: Wed Sep 30, 2020 9:42 pm
Re: Avogadro constant
You would want to use Avogadro's constant when converting moles into atoms/electrons/particles. You would also conversely use when converting from atoms/electrons/particles to moles.
-
Sarah Hong 2K
- Posts: 102
- Joined: Fri Sep 24, 2021 5:48 am
Re: Avogadro constant
You use Avogadro's constant when converting atoms to moles or moles to atoms. 1 mole is equivalent to Avogadro constant which is 6.022*10^23. So you can use this proportion when trying to convert atoms to moles or moles to atoms.
-
Coraly De Leon
- Posts: 105
- Joined: Fri Sep 24, 2021 5:56 am
Re: Avogadro constant
Hey,
You use Avogrado's number (6.02214 \times 10^{23}\, mol^{-1} when you are converting from moles to atoms or vice versa!
You use Avogrado's number (6.02214 \times 10^{23}\, mol^{-1} when you are converting from moles to atoms or vice versa!
-
Hannah Carsey 1B
- Posts: 100
- Joined: Fri Sep 24, 2021 6:05 am
Re: Avogadro constant
Avogadro's constant is the number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.022 × 10^23. The units may be electrons, atoms, ions, or molecules, depending on the nature of the substance. You use Avogadro's constant in stoichiometry when converting the moles of a substance into any "thing," such as atoms, ions, or molecules.
Re: Avogadro constant
You use Avogadro's constant whenever you need to convert from atoms/molecules/formula units to mol or vice versa.
-
Olivia Ghorai 1J
- Posts: 53
- Joined: Fri Sep 24, 2021 7:35 am
Re: Avogadro constant
You use Avogadro's constant whenever you a) have an amount in moles and wish to convert said amount to a unit like atoms, molecules, ions, etc and b) if you have an amount of something in a unit like atoms, molecules, ions etc and wish to convert this amount to moles. Avogadro's constant is 6.022 x 10^23, and the official definition of a mole is 6.022 x 10^23 particles (particles is a stand in for other units, you could technically have a mole of donuts).
If you are trying to convert from moles to the unit of particles you are dealing with, you'll want to multiply by Avogadro's constant, and if you are trying to convert from a unit of particles to moles you will want to divide by Avogadro's constant.
If you are trying to convert from moles to the unit of particles you are dealing with, you'll want to multiply by Avogadro's constant, and if you are trying to convert from a unit of particles to moles you will want to divide by Avogadro's constant.
-
Justin_Choo_3J
- Posts: 74
- Joined: Fri Sep 24, 2021 7:28 am
- Been upvoted: 4 times
- Contact:
Re: Avogadro constant
Avogadro's number (6.022 x 10^23) equates to one mole; the standard unit of measure for quantifying extremely small matter such as atoms, ions, and molecules. As such, Avogadro's number and its relationship with the mole put large quantities such as the number of atoms in a solution, into a manageable perspective. It is important to recognize that this concept is not only limited to the measurement of individual elements but can also be applied to compounds as well (e.g. 1 mole of CO2 = 6.022 x 10^23 units of CO2; 1 mole of C = 6.022 x 10^23 units of C).
-
Jordyn Lee 1J
- Posts: 106
- Joined: Fri Sep 24, 2021 7:08 am
Re: Avogadro constant
What is the difference between Avogadro's number and Avogadro constant? And for Avogadro's constant, is it safe to round it to 6.022x10^23 for every problem?
-
Ellen Tsai 3D
- Posts: 55
- Joined: Fri Sep 24, 2021 5:55 am
Re: Avogadro constant
You can use Avogadro's Constant whenever you need to find the number of molecules/atoms of a given element. You can multiply a given mole by 6.022 x10^23 to get the number of atoms/molecules or do the opposite so for example if you were given a number of atoms but needed to find the moles you divide by 6.022 x10^23. Hope this is helpful!
-
Phillip Ma 1J
- Posts: 100
- Joined: Fri Sep 24, 2021 7:05 am
Re: Avogadro constant
You use Avogadro's constant when you want to find the amount of atoms, formula units, or molecules. There are 6.022e23 atoms/formula units/molecules in 1 mol of whatever you have.
-
Triston Dinh 1D
- Posts: 101
- Joined: Fri Sep 24, 2021 6:03 am
Re: Avogadro constant
You would want to use Avogadro's constant when calculating the number of atoms and/or molecules of an element. There are 6.022*10^23 atoms//molecules in 1 mol of any element.
-
Jiayin Yola Yan 1G
- Posts: 60
- Joined: Fri Sep 24, 2021 6:20 am
Re: Avogadro constant
If the questions ask you to find the numbers of the "thing," could be an atom or a molecule, then you should use the avogadro constant. Keep in mind that it is a conversion between moles and the actual number of the object.
-
Alejandro Oliva 2F
- Posts: 50
- Joined: Fri Sep 24, 2021 5:39 am
Re: Avogadro constant
Avogadro's Constant is essentially Avogadro's number given a quantifiable unit of measure. In this case, the constant accounts for the number of "things" (atoms, molecules, other units) found in one mole of any given substance. The constant is commonly depicted as NA = 6.02214076×1023 mol−1.
-
Eric Sun 2G
- Posts: 102
- Joined: Fri Sep 24, 2021 5:08 am
Re: Avogadro constant
Any time that you are asked to do a unit conversion through a certain number of atoms/molecules, Avogadro's number will almost always be used. Often times, we use Avogadro's number as a placeholder to convert the large number of atoms to a smaller, easier to write mole conversion.
-
Matthew Li 1B
- Posts: 118
- Joined: Fri Sep 24, 2021 6:40 am
-
Alex_Lee_1K
- Posts: 105
- Joined: Fri Sep 24, 2021 5:26 am
Re: Avogadro constant
Avogadros constant is used to convert from mols of something to atoms or the other way around. If you are given, for example, 1.29x10^24 hydrogen atoms you would simply just have to divide by avagadro's constant (6.022x10^23 mol-1) in order to find how many mols of hydrogen you have. A good equation that I use came out of the textbook which is "N=nNa" where N stands for the number of atoms, n stands for the amount of mols, and Na stands for avagadro's constant.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Avogadro constant
Hello Savannah,
With respect to Avogadro's constant (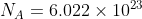 ), it is useful when you are attempting to convert into a mole of a substance, such as singular atoms, diatomic atoms, molecules, formula units, or electrons just to name a few.
), it is useful when you are attempting to convert into a mole of a substance, such as singular atoms, diatomic atoms, molecules, formula units, or electrons just to name a few.
With respect to Avogadro's constant (
-
Gabby Sigal 3H
- Posts: 49
- Joined: Fri Sep 24, 2021 6:43 am
Re: Avogadro constant
Considering that Avogardo's constraint is basically the amount of "objects"/ units in one mole of an object. Therefore, Avogadro's constant can be used to convert between the number of atoms and moles (as one mole of a specific compound or element is equal to 6.022x10^23 atoms of that compound/element). If you are given the mass of a sample of some sort of compound, you can convert the mass of the sample into moles (using n=m/M) and then convert from moles to atoms using Avogadro's number by multiplying n(number of moles) x 6.022*10^23
-
Valerie M Dis 2E
- Posts: 84
- Joined: Fri Sep 24, 2021 6:40 am
Re: Avogadro constant
Avagadro’s constant is 6.022 x10-23 atoms. So you would want to use it when converting from moles to atoms or when converting from atoms to moles.
-
Sidharth Paparaju 3B
- Posts: 167
- Joined: Fri Sep 24, 2021 5:14 am
Re: Avogadro constant
You use Avogadro's number to figure out how many formula units (atoms, molecules, etc.) are in x moles of a substance.
Hope that helps!
Hope that helps!
-
Heba Bounar 3K
- Posts: 100
- Joined: Fri Sep 24, 2021 5:49 am
Re: Avogadro constant
Hi! If you want to find atoms from moles, use Avogadro's constant with the units atoms/mole to (6.022*1O^23 atoms/mole) to find the number of atoms. To find molecules from moles, you use Avogadro's constant but with molecules/mole as the units.
Hope this helps!
Hope this helps!
-
Harsimer Bal 3K
- Posts: 106
- Joined: Fri Sep 24, 2021 5:16 am
- Been upvoted: 1 time
Re: Avogadro constant
Avogadro's constant (6.022x10^23) is used whenever moles need to be converted to formula units, atoms, photons, particles (etc.) or vice versa.
-
Cory Poon 3G
- Posts: 101
- Joined: Fri Sep 24, 2021 5:01 am
Re: Avogadro constant
Avogadro's number, 6.022 x 10^23 indicates that there are 6.022 x 10^23 molecules/particles in one mole of a substance. You'll use the constant when you need to find the number of molecules or particles.
-
Hannah Kline 3C
- Posts: 5
- Joined: Fri Sep 24, 2021 6:59 am
Re: Avogadro constant
Hi!! You'd use Avogadro's number when converting between number of molecules/mass/moles. It just makes comparing very large numbers much simpler!
-
Libby Stafford 1L
- Posts: 107
- Joined: Fri Sep 24, 2021 6:27 am
Re: Avogadro constant
Yeah! I think like what others have said, you need to multiply the number of moles of a substance/element etc by Avogadro's constant if they ask you to find the actual number of atoms present, as 1 mole of any substance has the same number of atoms as Avogadro's constant. You shouldn't need to do it though unless they ask you for number of atoms or mention the constant.
Return to “Accuracy, Precision, Mole, Other Definitions”
Who is online
Users browsing this forum: No registered users and 2 guests

