E15
Moderators: Chem_Mod, Chem_Admin
-
Samantha Loc 1B
- Posts: 103
- Joined: Fri Sep 24, 2021 5:03 am
E15
Question E15 states: "The molar mass of the metal hydroxide M(OH)2 is 74.10 g/mol. What is the molar mass of the sulfide of this metal?" I understand the basics of finding molar mass but wasn't quite sure what they meant when asking about the "sulfide of this metal". Does anyone know what "sulfide" would be considered/how to find it in this situation?
-
William Huang 1K
- Posts: 111
- Joined: Fri Sep 24, 2021 7:35 am
Re: E15
Hi,
Sulfide is the S-2 ion, and since you can tell that the oxidation number of the M metal in this hydroxide is +2, the Sulfide would be MS, so you would subtract two molar masses of hydroxide ion (OH-) from the molar mass and add the molar mass of a sulfur atom to get the molar mass of the metal sulfide.
Sulfide is the S-2 ion, and since you can tell that the oxidation number of the M metal in this hydroxide is +2, the Sulfide would be MS, so you would subtract two molar masses of hydroxide ion (OH-) from the molar mass and add the molar mass of a sulfur atom to get the molar mass of the metal sulfide.
-
amara ajon 1d
- Posts: 109
- Joined: Fri Sep 24, 2021 6:34 am
Re: E15
Hi,
The sulfide of M(OH)2 would basically replace the hydroxide component of the molecule with however many sulfur atoms are needed to create a metal sulfide. In other words, you would be finding the molar mass of MSx (where x is the number of S atoms in the molecule).
The sulfide of M(OH)2 would basically replace the hydroxide component of the molecule with however many sulfur atoms are needed to create a metal sulfide. In other words, you would be finding the molar mass of MSx (where x is the number of S atoms in the molecule).
-
Crystal Ma 2J
- Posts: 100
- Joined: Fri Sep 24, 2021 7:04 am
Re: E15
In this case, the sulfide would be MS. In this case, to find the molar mass of the sulfide of this metal, you would first have to subtract the molar mass of (OH)2 from 74.10 g/mol. That gives you the molar mass of M, which you would then add to the molar mass of a sulfur atom.
-
Samantha Loc 1B
- Posts: 103
- Joined: Fri Sep 24, 2021 5:03 am
Re: E15
amara ajon 1e wrote:Hi,
The sulfide of M(OH)2 would basically replace the hydroxide component of the molecule with however many sulfur atoms are needed to create a metal sulfide. In other words, you would be finding the molar mass of MSx (where x is the number of S atoms in the molecule).
Hi, thanks for the feedback!
So after subtracting the two hydroxides since they are replaced by the sulfides, how would you know that only one sulfur atom needs to be added (this would just entail adding the molar mass of sulfur right?)?
-
amara ajon 1d
- Posts: 109
- Joined: Fri Sep 24, 2021 6:34 am
Re: E15
Samantha Loc 1B wrote:amara ajon 1e wrote:Hi,
The sulfide of M(OH)2 would basically replace the hydroxide component of the molecule with however many sulfur atoms are needed to create a metal sulfide. In other words, you would be finding the molar mass of MSx (where x is the number of S atoms in the molecule).
Hi, thanks for the feedback!
So after subtracting the two hydroxides since they are replaced by the sulfides, how would you know that only one sulfur atom needs to be added (this would just entail adding the molar mass of sulfur right?)?
Since we know that S is typically 2-, and we already know that M is 2+ (since we had two hydroxide ions, each with -1), we would get MS as the metal sulfide.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: E15
Hello Samantha,
When approaching this problem you would cite the corresponding sulfide ion (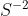 ) and note the corresponding charge. Once the metal (
) and note the corresponding charge. Once the metal (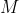 ) is deciphered, ensure the charges and amounts are equivalent (1+ for every 1-) to ensure neutrality. Finally, refer to the periodic table for molar mass determination.
) is deciphered, ensure the charges and amounts are equivalent (1+ for every 1-) to ensure neutrality. Finally, refer to the periodic table for molar mass determination.
When approaching this problem you would cite the corresponding sulfide ion (
Return to “Accuracy, Precision, Mole, Other Definitions”
Who is online
Users browsing this forum: No registered users and 6 guests

