The nuclear power industry extracts 6Li but not 7Li from natural samples of lithium. As a result, the molar mass of commercial samples of lithium is increasing. The current abundances of the two isotopes are 7.42% and 92.58%, respectively, and the masses of their atoms are 9.988*10^-24 and 1.165*10^-23 respectively. (A) what is the current molar mass of a natural sample of lithium? (B) what will the molar mass be when the abundance of 6Li is reduced to 5.67?
Can someone just walk me through the process of solving this. Thank you.
Fundamental E11
Moderators: Chem_Mod, Chem_Admin
-
Anne Cam 3A
- Posts: 71
- Joined: Fri Sep 25, 2015 3:00 am
Re: Fundamental E11
To find the average molar mass of an element from the percentage of its isotopes, you would multiply the percentages (in decimals) by their respective molar masses.
In (a), only the masses of 6Li and 7Li atoms are given, so you would need to convert them to g/mol. Multiply each mass value by 6.022*10^23, the number of atoms in a mole, to get the molar mass for each Li isotope. (About 6.0148 g/mol for 6Li, and 7.0156 g/mol for 7Li)
Then multiply each molar mass by its corresponding percentage, and add the results to get the average molar mass. It would look like this:
(0.0742*6.0148) + (0.9258*7.0156) = 6.94 g/mol (rounded with 2 significant figures)
For part (b), you would keep the molar mass values for each isotope, but change the percentage of 6Li to 5.67%, and again multiply each molar mass by its corresponding percentage to get the average molar mass. The process is the same, except one percentage is changed.
Hope this helps!
In (a), only the masses of 6Li and 7Li atoms are given, so you would need to convert them to g/mol. Multiply each mass value by 6.022*10^23, the number of atoms in a mole, to get the molar mass for each Li isotope. (About 6.0148 g/mol for 6Li, and 7.0156 g/mol for 7Li)
Then multiply each molar mass by its corresponding percentage, and add the results to get the average molar mass. It would look like this:
(0.0742*6.0148) + (0.9258*7.0156) = 6.94 g/mol (rounded with 2 significant figures)
For part (b), you would keep the molar mass values for each isotope, but change the percentage of 6Li to 5.67%, and again multiply each molar mass by its corresponding percentage to get the average molar mass. The process is the same, except one percentage is changed.
Hope this helps!
-
Nicolette_Canlian_2L
- Posts: 77
- Joined: Fri Sep 28, 2018 12:25 am
- Been upvoted: 1 time
Re: Fundamental E11
The method I used to solve part a is by multiplying the mass of each with its correlating percentage of abundance and then added these products together.
m=0.0742(9.988x10^-24g)+0.9258(1.165x10^-23g)
m=1.153x10^-23g
Then I multiplied the sum of those two with Avogadro's constant and got 6.94g/mol
m=0.0742(9.988x10^-24g)+0.9258(1.165x10^-23g)
m=1.153x10^-23g
Then I multiplied the sum of those two with Avogadro's constant and got 6.94g/mol
-
Christian_Lee_2K
- Posts: 174
- Joined: Fri Sep 29, 2023 12:02 pm
Re: Fundamental E11
Hi there!
I wanted clarification as to why we would add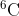 and
and 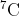 in part a. I thought we would only use
in part a. I thought we would only use 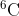 because it is asking for the natural sample and only
because it is asking for the natural sample and only 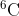 is pulled from a natural sample.
is pulled from a natural sample.
I wanted clarification as to why we would add
Return to “Accuracy, Precision, Mole, Other Definitions”
Who is online
Users browsing this forum: No registered users and 6 guests

