Effective nuclear charge,
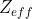
, describes the nuclear charge experienced by an electron (we usually focus on the valence electrons when talking about it), and is often less than the actual nuclear charge because it accounts for electron-electron repulsion.
Take for example, Carbon, which has 6 protons. Lets suppose that Carbon has a nuclear charge of +6. Carbon's valence electrons don't experience the full force of attraction to this +6 charge. The negative charges of non-valence electrons closer to carbon's nucleus negate the positive attraction that binds valence electrons to the nucleus. In term's of Coloumb's law and electrostatic forces, this manifests as inner electrons pushing outwards against valence electrons attracted to the nucleus. The push outwards thus negates a portion of the pull inwards experienced by valence electrons. We call this shielding. Note that valence electrons within the same shell shield each other much less from the inwards pull.
So then what would a
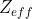
look like for a Carbon valence electron? The general equation is
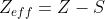
, where Z is the atomic number and S is the shielding strength value. For simplicity's sake, lets assume each non-valence electron contributes a shielding strength value of 1.This makes Z = 6 and S = 2 for Carbon (1 per electron in the n=1 shell. The electrons in n=2 shell are valence electrons). Thus
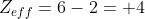
for Carbon.