Question 1.15 on homework
Moderators: Chem_Mod, Chem_Admin
-
Sidney Kantono 3C
- Posts: 1
- Joined: Fri Sep 25, 2015 3:00 am
Question 1.15 on homework
"In the ultraviolet spectrum of atomic hydrogen, a line is observed at 102.6 nm. Determine the values of n for the initial and final energy levels of the electron during the emission of energy that leads to this spectral line."
Can anyone explain how to do this question? I noticed that there was an earlier post about this, but the answer to it still did not clarify it for me. Thank you!
Can anyone explain how to do this question? I noticed that there was an earlier post about this, but the answer to it still did not clarify it for me. Thank you!
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Question 1.15 on homework
The fact that the emission is ultraviolet tells you that this transition is in the Lyman series, so n2=1
Convert 102.6 nm into the energy E using the two relations: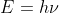 and
and 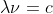
Then plug into the rydberg formula and solve for n1
Convert 102.6 nm into the energy E using the two relations:
Then plug into the rydberg formula and solve for n1
-
Hank Wang 2J
- Posts: 25
- Joined: Fri Sep 25, 2015 3:00 am
-
Alex Nguyen 3I
- Posts: 100
- Joined: Fri Sep 25, 2015 3:00 am
Re: Question 1.15 on homework
Spectral lines in the same series always end with the same n2 or final energy level. The Lyman series happens to contain all energy transitions that end with n=1. The initial energy level could be any energy level greater than 1 though. For the hydrogen atom, the electron that drops to n=1 will emit UV radiation that can range from any of the lines/wavelengths in the Lyman series.
-
Alex Nguyen 3I
- Posts: 100
- Joined: Fri Sep 25, 2015 3:00 am
Re: Question 1.15 on homework
According to the Rydberg Formula, n1 is actually the final energy level I guess you could say. Therefore solving will only work if you designate n1 as being equal to 1. That's just the way the formula is set up. I'm pretty sure n=1 is still the final energy level though.
-
Ana Pedreros
- Posts: 62
- Joined: Fri Sep 28, 2018 12:19 am
Re: Question 1.15 on homework
My way to approach this problem was to:
1. figure out the frequency using the wavelength of 102.6 nm and the formula v=c/lambda
2. then use the frequency and plug it into Rydberg formula
3. since the problem states its in the UV spectrum the initial energy should be 1 leaving n2 as the only undefined variable
4. distribute the R constant and solve for n2 and you should get 3
1. figure out the frequency using the wavelength of 102.6 nm and the formula v=c/lambda
2. then use the frequency and plug it into Rydberg formula
3. since the problem states its in the UV spectrum the initial energy should be 1 leaving n2 as the only undefined variable
4. distribute the R constant and solve for n2 and you should get 3
-
Nick Lewis 4F
- Posts: 111
- Joined: Wed Sep 18, 2019 12:18 am
Re: Question 1.15 on homework
I had a bit of trouble with this problem at first, but I looked at the problem on pg.7&8 in the textbook as an example. It goes from n1 and n2 to the wavelength of the emitted radiation. My only trouble in going back was how do we know n1=1? Otherwise it was easy to use the equations c = lambda * v and v = R(1/n1^2-1/n2^2)
-
Justin Quan 4I
- Posts: 104
- Joined: Sat Sep 14, 2019 12:17 am
Re: Question 1.15 on homework
Nick Lewis 3G wrote:I had a bit of trouble with this problem at first, but I looked at the problem on pg.7&8 in the textbook as an example. It goes from n1 and n2 to the wavelength of the emitted radiation. My only trouble in going back was how do we know n1=1? Otherwise it was easy to use the equations c = lambda * v and v = R(1/n1^2-1/n2^2)
You know n1=1 because the problem tells you the that it was an ultraviolet wave. Ultraviolet has a n value of 1
-
Camille 4I
- Posts: 57
- Joined: Sat Aug 24, 2019 12:18 am
Re: Question 1.15 on homework
How can you tell that the transition is in the Lyman series from the fact that it is in the ultraviolet spectrum of atomic hydrogen?
Re: Question 1.15 on homework
When the question says that it is ultraviolet, we know that the line is observed at n=1. If there is a final n value of 3, it is paschen series, if it is 2 it is balmer and if it is 1 it is lynman. Because n final is 1, we can then say that it is lynman series.
-
Aman Sankineni 2L
- Posts: 103
- Joined: Fri Aug 30, 2019 12:17 am
- Been upvoted: 1 time
Re: Question 1.15 on homework
Camille 4I wrote:How can you tell that the transition is in the Lyman series from the fact that it is in the ultraviolet spectrum of atomic hydrogen?
Lyman series are the only series found in the ultraviolet spectrum of atomic hydrogen. By knowing that the transition is in the ultraviolet spectrum allows us to know that it is a Lyman series.
-
Camille 4I
- Posts: 57
- Joined: Sat Aug 24, 2019 12:18 am
Re: Question 1.15 on homework
How would you solve this problem using the equation En = -hR/n^2? This is the equation included on the Constants and Equations sheet.
Re: Question 1.15 on homework
Does it matter which one is n1 and which one is n2? Is it always the bigger number that is n2?
-
Sally_Luo_3F
- Posts: 85
- Joined: Fri Sep 24, 2021 5:29 am
Re: Question 1.15 on homework
Camille 4I wrote:How would you solve this problem using the equation En = -hR/n^2? This is the equation included on the Constants and Equations sheet.
I don't think you can solve the problem using this equation because this equation is calculating for the energy of the atom which is not what we want.
Re: Question 1.15 on homework
Nick Lewis 4F wrote:I had a bit of trouble with this problem at first, but I looked at the problem on pg.7&8 in the textbook as an example. It goes from n1 and n2 to the wavelength of the emitted radiation. My only trouble in going back was how do we know n1=1? Otherwise it was easy to use the equations c = lambda * v and v = R(1/n1^2-1/n2^2)
All transitions in the Hydrogen atom from n > 1 to n = 1 emit radiation in the UV region, this series of transitions to n =1 are known as the Lyman series. Since the question states that the emitted radiation was in the UV region, we know that the final state of the electron must be n = 1.
-
Edwin Montalvo 1G
- Posts: 113
- Joined: Fri Sep 24, 2021 6:27 am
Re: Question 1.15 on homework
It is important to know the Lyman series and the Balmer series. The Lyman series always ends in the final energy level of n=1, which is going to be in the UV region. The Balmer series ends in the final energy level of n=2, which is in the visible region. Knowing this allows you to calculate the change in energy from one energy level to the next while knowing what type of electromagnetic radiation will be emitted. Hope this helps!
-
Nathalia Garibay 1D
- Posts: 107
- Joined: Fri Sep 24, 2021 7:04 am
Re: Question 1.15 on homework
sophiavmr wrote:Does it matter which one is n1 and which one is n2? Is it always the bigger number that is n2?
Contrary to how it appears, the bigger number is not always n2. N2 represents the final position of the electron. So if the electron is excited from a lower orbital to a higher orbital, then n2 would be bigger. However, if the electron is dropping from a higher energy level to a lower energy level, then n2 would actually be the lower number.
Hope this helps!
Return to “Properties of Electrons”
Who is online
Users browsing this forum: No registered users and 4 guests

