Please can someone do a step by step method for the achieve #10 question:
The electron in a hydrogen atom is excited to the n= 5
shell and emits electromagnetic radiation when returning to lower energy levels. Determine the number of spectral lines that could appear when this electron returns to the lower energy levels, as well as the wavelength range in nanometers.
Thanks.
Achieve #10
Moderators: Chem_Mod, Chem_Admin
-
Ethan Famas 1H
- Posts: 100
- Joined: Fri Sep 24, 2021 5:57 am
Re: Achieve #10
The number of spectral lines can be found when you count all the possible movements of the electron: for each you start at n = 5 and end at n = each number until 1... so the ending possibilities are n = 4, 3, 2, 1.
The wavelength range can be found by using the equations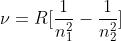 and
and 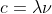 , where R is the Rydberg constant).
, where R is the Rydberg constant).
For the first equation, by plugging in n = 4 for n1 and n = 5 for n2, you would get the lowest frequency (which would mean the longest wavelength). You can also infer that by plugging in n = 1 for n1 and n = 5 for n2, you would get the highest frequency (shortest wavelength).
Then, you can use the second equation to convert frequencies to wavelengths. Pay attention to the units too, which are given in nm.
The wavelength range can be found by using the equations
For the first equation, by plugging in n = 4 for n1 and n = 5 for n2, you would get the lowest frequency (which would mean the longest wavelength). You can also infer that by plugging in n = 1 for n1 and n = 5 for n2, you would get the highest frequency (shortest wavelength).
Then, you can use the second equation to convert frequencies to wavelengths. Pay attention to the units too, which are given in nm.
Last edited by Ethan Famas 1H on Sun Oct 24, 2021 10:41 pm, edited 2 times in total.
-
Benicio Rivera 1F
- Posts: 138
- Joined: Fri Sep 24, 2021 6:42 am
Re: Achieve #10
Here is how I did mine. My problem had n=7 instead of n=5.
The electron that is excited to the n=7 energy level can transition to the 6,5,4,3,2, and 1 energy levels. Therefore, there are a total of six possible transitions that will produce six distinct spectral lines.
The range of wavelengths produced will be from the highest‑energy transition (n=7 to n=1) to the lowest‑energy transition (n=7 to n=6). The frequencies of these transitions can be calculated by using the equation,
(frequency) = RH(1/n^2_2 − 1/n^2_1) This means 1/(lower energy level) squared - 1/(higher energy level) squared
RH is the Rydberg constant, 3.29×1015 s−1.
The calculation for the frequency of the n=7 to n=1 transition, which is highest in energy, is shown.
(frequency_7,1) = 3.29×10^15s^−1 (1/1^2−1/7^2) = 3.22×10^15s^−1
Then, convert this frequency to wavelength in meters using the equation,
(Wave length_7,1)= c/(frequency_7,1)
where c is the speed of light (3.00×10^8 m/s). Recall that there are 10^−9 m in 1 nm.
(Wave length_7,1)= (3.00×10^8m/s)/ (3.22×10^15s^−1) =9.31×10^−8 m
(Wave length_7,1)= 9.31×10^−8 m(1nm/10^−9 m)= 93.1 nm
The calculation of the wavelength of energy emitted by the n=7 to n =6 transition, which is the lowest‑energy transition, is performed in the same way.
(frequency_7,6)= 3.29×10^15 s−1(1/6^2−1/7^2) = 2.42×10^13s^−1
(Wave length_7,6)= (3.00×10^8 m/s)/ 2.42×10^13s^−1 = 1.24×10^−5 m
(Wave length_7,6)= 1.24×10^−5 m (1nm/10^−9 m) = 12400 nm
Therefore, the wavelength range of possible spectral lines is 93.1 nm to 12400 nm.
The electron that is excited to the n=7 energy level can transition to the 6,5,4,3,2, and 1 energy levels. Therefore, there are a total of six possible transitions that will produce six distinct spectral lines.
The range of wavelengths produced will be from the highest‑energy transition (n=7 to n=1) to the lowest‑energy transition (n=7 to n=6). The frequencies of these transitions can be calculated by using the equation,
(frequency) = RH(1/n^2_2 − 1/n^2_1) This means 1/(lower energy level) squared - 1/(higher energy level) squared
RH is the Rydberg constant, 3.29×1015 s−1.
The calculation for the frequency of the n=7 to n=1 transition, which is highest in energy, is shown.
(frequency_7,1) = 3.29×10^15s^−1 (1/1^2−1/7^2) = 3.22×10^15s^−1
Then, convert this frequency to wavelength in meters using the equation,
(Wave length_7,1)= c/(frequency_7,1)
where c is the speed of light (3.00×10^8 m/s). Recall that there are 10^−9 m in 1 nm.
(Wave length_7,1)= (3.00×10^8m/s)/ (3.22×10^15s^−1) =9.31×10^−8 m
(Wave length_7,1)= 9.31×10^−8 m(1nm/10^−9 m)= 93.1 nm
The calculation of the wavelength of energy emitted by the n=7 to n =6 transition, which is the lowest‑energy transition, is performed in the same way.
(frequency_7,6)= 3.29×10^15 s−1(1/6^2−1/7^2) = 2.42×10^13s^−1
(Wave length_7,6)= (3.00×10^8 m/s)/ 2.42×10^13s^−1 = 1.24×10^−5 m
(Wave length_7,6)= 1.24×10^−5 m (1nm/10^−9 m) = 12400 nm
Therefore, the wavelength range of possible spectral lines is 93.1 nm to 12400 nm.
-
Zoe Apple 1F
- Posts: 50
- Joined: Fri Sep 24, 2021 5:38 am
Re: Achieve #10
For the first part of the problem, I knew that there were four spectral lines because it could go to 4, 3, 2, and 1 (four options). The second part requires you to use the Rydberg constant to find the frequency and then convert to wavelength using lambda=c/v. Make sure n2 is the lower energy level and n1 is the higher energy level.
Return to “Properties of Electrons”
Who is online
Users browsing this forum: No registered users and 4 guests

