2A.17 Predict the number of valence electrons present for each of the following ions:
a.) Mn^4+
I need help understanding why the answer is 3 valence electrons in the answer section. Because I saw a youtube video saying we count all the electrons of the highest "n" value in the electron configuration and those would be the # of valence electrons.
So for the electron configuration of Mn^4+ I got (1s^2)(2s^2)(2p^6)(3s^2)(3p^6)(3d^3) and since the highest n value is 3, i thought we count all the electrons of all the n values of 3, which I thought would be 11?
is it because I might've done the electron configuration wrong?
textbook problem from 2A #17
Moderators: Chem_Mod, Chem_Admin
-
Sofia Lucido 3L
- Posts: 112
- Joined: Wed Sep 30, 2020 9:51 pm
- Been upvoted: 1 time
Re: textbook problem from 2A #17
I think that the electrons in 3s and 3p are not considered valence electrons even though they are in the n=3 state. If you shorthand the electron configuration and write 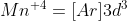 then it becomes more clear that those electrons in the 3s and 3p subshells are considered to be part of the core electrons. I'm not totally sure why, I think it might be because they have lower energy? It's a good question!
then it becomes more clear that those electrons in the 3s and 3p subshells are considered to be part of the core electrons. I'm not totally sure why, I think it might be because they have lower energy? It's a good question!
-
Claire_Latendresse_1E
- Posts: 143
- Joined: Wed Sep 30, 2020 9:37 pm
- Been upvoted: 2 times
Re: textbook problem from 2A #17
Mn's electron configuration in its ground state is [Ar](3d^5)(4s^2), so it has 7 valence electrons in its ground state. The Mn^4+ ion's configuration is [Ar](3d^3), so it has 3 valence electrons. For me, it's helpful to write the electron configurations with the noble gas substitution ([Ar] in this case) because it makes it clear which electrons are the valence electrons.
I would be a bit careful with the n value trick you talked about because of how funky the 3d orbital is (lower energy level than 4s).
I hope this helps a bit :)
I would be a bit careful with the n value trick you talked about because of how funky the 3d orbital is (lower energy level than 4s).
I hope this helps a bit :)
Return to “Ionic & Covalent Bonds”
Who is online
Users browsing this forum: No registered users and 12 guests

