Nonpolar vs Polar covalent
Moderators: Chem_Mod, Chem_Admin
-
Melody Haratian 2J
- Posts: 125
- Joined: Wed Sep 30, 2020 9:48 pm
- Been upvoted: 2 times
Nonpolar vs Polar covalent
Hi guys! So covalent bonds are polar when the electrons are shared unequally and they’re nonpolar when the electrons are shared equally. Usually, how close do the atoms have to be in electronegativity for a bond to be considered nonpolar.
Also, are C-H bonds considered nonpolar?
Thanks!
Also, are C-H bonds considered nonpolar?
Thanks!
-
Nathan Lao 2I
- Posts: 100
- Joined: Wed Sep 30, 2020 9:40 pm
- Been upvoted: 1 time
Re: Nonpolar vs Polar covalent
I think a difference of 0.4 or less leads to nonpolar covalent bonding. Following that rule, a C-H bond would be nonpolar.
-
Joseph Liao 3C
- Posts: 55
- Joined: Tue Sep 28, 2021 5:04 am
Re: Nonpolar vs Polar covalent
In my experience, there are a few polar bonds that commonly come up (especially in biology), when C is bonded with either O and N. If anything, it could be helpful to just remember that those bonds are polar, and it could get you through a majority of questions. The C-H bond is non-polar.
-
Shreya Arcot 3K
- Posts: 101
- Joined: Fri Sep 24, 2021 6:03 am
Re: Nonpolar vs Polar covalent
It's useful to remember this in terms of common atoms. Oxygen and nitrogen atoms have high electronegativities. Carbon and hydrogen atoms have low electronegativities. So, a C-H bond is nonpolar. C bonded to either O or N would be polar, and H bonded to either O or N would be polar as well.
-
Desiree Eshraghi 3A
- Posts: 136
- Joined: Fri Sep 24, 2021 6:12 am
Re: Nonpolar vs Polar covalent
C-H is nonpolar. When it comes to figuring this out I tend to look out for O or N as I was taught they were "greedy" elements that love to steal their bonded's electronegativity. C and H on the other hand do not have a high electronegativity.
-
Wenhan Li_3d
- Posts: 50
- Joined: Wed Feb 17, 2021 12:15 am
-
Aparna Pillai 1E
- Posts: 109
- Joined: Fri Sep 24, 2021 7:09 am
Re: Nonpolar vs Polar covalent
Generally the more electronegative element will attract electrons closer to itself in a molecule. Because C and H both have low electronegativity with a small difference between them, C-H is a nonpolar bond. You can usually compare electronegativity using the rule that electronegativity usually increases going right across periods and up across groups on the periodic table.
-
Lynne Xu 3K
- Posts: 102
- Joined: Fri Sep 24, 2021 6:08 am
Re: Nonpolar vs Polar covalent
If two atoms have an electronegativity difference of between 0.5-1.7 (the numbers vary from source to source), it will be a polar covalent bond. If the electronegativity difference is less than 0.5, the bond can generally be considered nonpolar.
Carbon has an EN value of 2.55, while Hydrogen has an EN value of 2.2. Carbon-hydrogen bonds are non polar, as the difference of electronegativities is less than 0.5.
Carbon has an EN value of 2.55, while Hydrogen has an EN value of 2.2. Carbon-hydrogen bonds are non polar, as the difference of electronegativities is less than 0.5.
-
Sasha Gladkikh 2A
- Posts: 190
- Joined: Fri Sep 24, 2021 5:50 am
- Been upvoted: 3 times
Re: Nonpolar vs Polar covalent
Lynne Xu wrote:If two atoms have an electronegativity difference of between 0.5-1.7 (the numbers vary from source to source), it will be a polar covalent bond. If the electronegativity difference is less than 0.5, the bond can generally be considered nonpolar.
Carbon has an EN value of 2.55, while Hydrogen has an EN value of 2.2. Carbon-hydrogen bonds are non polar, as the difference of electronegativities is less than 0.5.
To add on, if the difference in the electronegativity between the two bonded atoms is greater than 1.7, then the bond is considered to be ionic. Because one atom pulls the other atom's electrons so well toward itself, there is a great imbalance of electric charge.
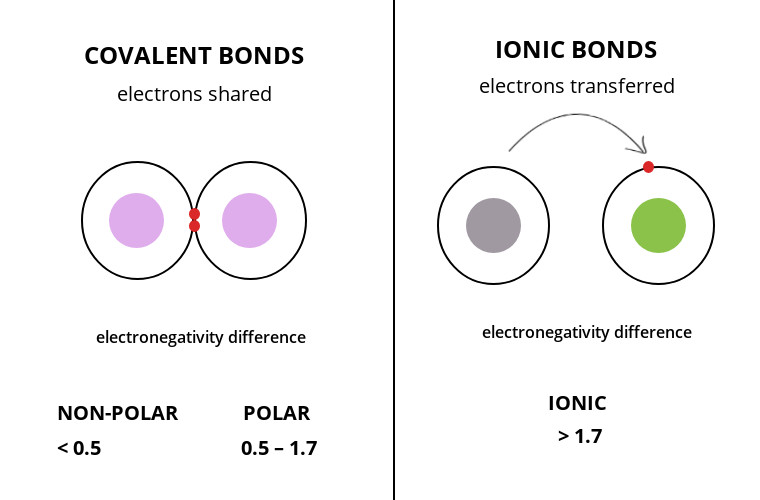
In summary,
Non-Polar Covalent: less than 0.5
Polar Covalent: between 0.5 and 1.7
Ionic: greater than 1.7
-
Theo Teske 3B
- Posts: 28
- Joined: Fri Sep 24, 2021 5:07 am
Re: Nonpolar vs Polar covalent
Sasha Gladkikh 1C wrote:Lynne Xu wrote:If two atoms have an electronegativity difference of between 0.5-1.7 (the numbers vary from source to source), it will be a polar covalent bond. If the electronegativity difference is less than 0.5, the bond can generally be considered nonpolar.
Carbon has an EN value of 2.55, while Hydrogen has an EN value of 2.2. Carbon-hydrogen bonds are non polar, as the difference of electronegativities is less than 0.5.
To add on, if the difference in the electronegativity between the two bonded atoms is greater than 1.7, then the bond is considered to be ionic. Because one atom pulls the other atom's electrons so well toward itself, there is a great imbalance of electric charge.
In summary,
Non-Polar Covalent: less than 0.5
Polar Covalent: between 0.5 and 1.7
Ionic: greater than 1.7
The ranges given are very helpful. I was wondering if this is a hard and fast rule (for example, is an ionic bond defined as electronegativity difference being greater than 1.7) or whether it's more of a useful rule of thumb. Are there some bonds that break these rules, like is there a non-polar covalent bond with electronegativity difference of 0.7, or is that not possible?
-
Matthew Nguyen 2F
- Posts: 103
- Joined: Fri Sep 24, 2021 7:35 am
Re: Nonpolar vs Polar covalent
Theo Teske 3B wrote:Sasha Gladkikh 1C wrote:Lynne Xu wrote:If two atoms have an electronegativity difference of between 0.5-1.7 (the numbers vary from source to source), it will be a polar covalent bond. If the electronegativity difference is less than 0.5, the bond can generally be considered nonpolar.
Carbon has an EN value of 2.55, while Hydrogen has an EN value of 2.2. Carbon-hydrogen bonds are non polar, as the difference of electronegativities is less than 0.5.
To add on, if the difference in the electronegativity between the two bonded atoms is greater than 1.7, then the bond is considered to be ionic. Because one atom pulls the other atom's electrons so well toward itself, there is a great imbalance of electric charge.
In summary,
Non-Polar Covalent: less than 0.5
Polar Covalent: between 0.5 and 1.7
Ionic: greater than 1.7
The ranges given are very helpful. I was wondering if this is a hard and fast rule (for example, is an ionic bond defined as electronegativity difference being greater than 1.7) or whether it's more of a useful rule of thumb. Are there some bonds that break these rules, like is there a non-polar covalent bond with electronegativity difference of 0.7, or is that not possible?
If anything I've learned about bonds, these rules are more like guidelines than they are absolute hard and fast rules. Ranges for these bonds being polar, nonpolar, or ionic vary all the time.
-
Peter Fernandez 2K
- Posts: 56
- Joined: Fri Sep 24, 2021 6:21 am
Re: Nonpolar vs Polar covalent
For determining if a bond is polar or nonpolar, it's important to think of difference in electronegativities. When two atoms are covalently bonded and have very different electronegativities (C-O for example) then the bond is polar. If the two atoms have similar electronegativities, like having two of the same atoms covalently bonded, it results in a nonpolar bond.
-
madelyn kelly 1I
- Posts: 53
- Joined: Fri Sep 24, 2021 6:24 am
Re: Nonpolar vs Polar covalent
You can typically find determine the type of bond depending on where the two atoms are found on the periodic table. The farther right and upward you move on the periodic table, the higher the electronegativity with a few exceptions of H being found to have a medium electronegativity being found on the far left, Mn has a low electronegativity despite being found in the middle range, and Au has a relatively high electronegativity despite being found in the middle electronegativity range. When there are two vastly different electronegativities, this will lead to unequal sharing of electrons.
-
gracebinder3I
- Posts: 102
- Joined: Fri Sep 24, 2021 5:43 am
- Been upvoted: 1 time
Re: Nonpolar vs Polar covalent
Hi!
For atoms to be considered to have a non-polar bond, they have a distance less than 0.4. Conversely, if the difference in electronegativities is greater than 0.4, the bond is polar. If the distance exceeds 1.8, the bond is ionic.
C-H bonds are considered non-polar.
I hope this helps!
For atoms to be considered to have a non-polar bond, they have a distance less than 0.4. Conversely, if the difference in electronegativities is greater than 0.4, the bond is polar. If the distance exceeds 1.8, the bond is ionic.
C-H bonds are considered non-polar.
I hope this helps!
-
Simone Byun 1F
- Posts: 101
- Joined: Fri Sep 24, 2021 5:58 am
- Been upvoted: 1 time
Re: Nonpolar vs Polar covalent
Very generally, if two atoms are both have a high or low electronegativity, they will be non polar. On the other hand, if one electronegativity is high and the other is low, is it likely to be polar. The exact difference is 0.4. Since the difference in electronegativity between C and H is <0.4, that bond is non polar.
Return to “Ionic & Covalent Bonds”
Who is online
Users browsing this forum: No registered users and 1 guest

