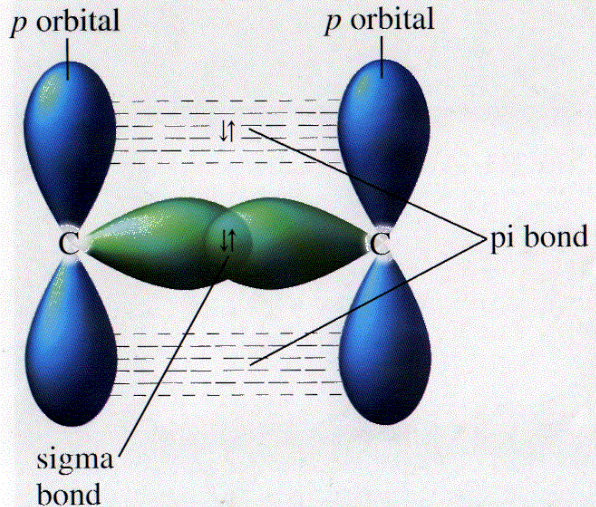
pi bonds
Moderators: Chem_Mod, Chem_Admin
-
Natalie 3k
- Posts: 102
- Joined: Wed Sep 30, 2020 10:11 pm
pi bonds
Hi! Does anyone have any tips for better visualizing how pi bonds work? I understand the basics but I'm having a hard time picturing them. Thank you!
-
SophiaNguyen_2L
- Posts: 112
- Joined: Wed Sep 30, 2020 9:59 pm
-
Jiwon_Chae_3L
- Posts: 99
- Joined: Wed Sep 30, 2020 9:39 pm
Re: pi bonds
The way that I understand it is that sigma bonds are connected linearly at the tips, and pi bonds are connected laterally, at the ends of 2 dumbbells.
-
reva_bajjuri
- Posts: 117
- Joined: Fri Oct 02, 2020 12:17 am
-
Hannah Biju 1E
- Posts: 108
- Joined: Wed Sep 30, 2020 9:55 pm
-
reva_bajjuri
- Posts: 117
- Joined: Fri Oct 02, 2020 12:17 am
-
Stephen Min 1I
- Posts: 100
- Joined: Wed Sep 30, 2020 10:01 pm
Re: pi bonds
A pi bond is formed by two p-orbitals overlapping side-by-side, as if two dumbbells were parallel to each other. This prevents the bound atoms from rotating.
-
Gigi Elizarraras 2C
- Posts: 100
- Joined: Wed Sep 30, 2020 9:41 pm
Re: pi bonds
Pi bonds are formed laterally so that the molecules cannot rotate. Sigma bonds are formed at the tip so that rotation is allowed. Basically pi bonds make the molecules stuck in position:)
-
Emily Jacobo 1C
- Posts: 100
- Joined: Wed Sep 30, 2020 9:37 pm
Re: pi bonds
I had a hard time too on understanding the two, thanks to all that explained it here. I'm more of a visual person and the visuals really helped me to understand clearer.
-
Moura Girgis 1F
- Posts: 109
- Joined: Wed Sep 30, 2020 10:00 pm
Re: pi bonds
Think of pi bonds as a vertical overlapping of bonds, so they cannot rotate, while sigma bonds consist of horizontal overlapping, in which they can rotate.
-
Daniela_Martinez_3B
- Posts: 51
- Joined: Wed Sep 30, 2020 9:47 pm
Re: pi bonds
The organic chemistry tutor on youtube has a great video on sigma and pi bonds that really helped me visualize them!
-
Kandyce Lance 3E
- Posts: 100
- Joined: Wed Sep 30, 2020 9:46 pm
Re: pi bonds
reva_bajjuri wrote:so for the above ethylene structure are all of the hydrogens in the same plane?
Yes thats correct!
-
Kandyce Lance 3E
- Posts: 100
- Joined: Wed Sep 30, 2020 9:46 pm
Re: pi bonds
Daniela_Martinez_3B wrote:The organic chemistry tutor on youtube has a great video on sigma and pi bonds that really helped me visualize them!
Do you mind sharing the link to the video that helped you?
-
Daria Obukhova 2B
- Posts: 100
- Joined: Wed Sep 30, 2020 9:50 pm
Re: pi bonds
Moura Girgis 1D wrote:Think of pi bonds as a vertical overlapping of bonds, so they cannot rotate, while sigma bonds consist of horizontal overlapping, in which they can rotate.
So, since they can't rotate, is that why you get atoms in different planes once you start adding to the central atom? Or is there another reason? I just remember a Sapling question we had about that.
-
Alan Nguyen 2I
- Posts: 100
- Joined: Fri Sep 24, 2021 6:43 am
Re: pi bonds
Daniela_Martinez_3B wrote:The organic chemistry tutor on youtube has a great video on sigma and pi bonds that really helped me visualize them!
I recommend the channel "The Organic Chemistry Tutor" as well! Many of his videos are extremely helpful in just detailing the step-by-step of difficult problems. It can be very beneficial to hear the same material taught from a different perspective.
-
Tristan Friet 3G
- Posts: 100
- Joined: Wed Feb 17, 2021 12:23 am
Re: pi bonds
Below is a link to a video done by "The Organic Chemistry Tutor" on Sigma and Pi bonds. Check it out!
https://www.youtube.com/watch?v=pT8nrBrTOm4
https://www.youtube.com/watch?v=pT8nrBrTOm4
-
Aashna Bhandari 1L
- Posts: 102
- Joined: Fri Sep 24, 2021 7:06 am
Re: pi bonds
A pi bond is two p-orbitals overlapping while side-by-side. You can visualize it as if two dumbbells were parallel next to each other.
Who is online
Users browsing this forum: No registered users and 5 guests