"side to side"
Moderators: Chem_Mod, Chem_Admin
-
Rohita Thammineni 2D
- Posts: 103
- Joined: Fri Sep 24, 2021 7:10 am
"side to side"
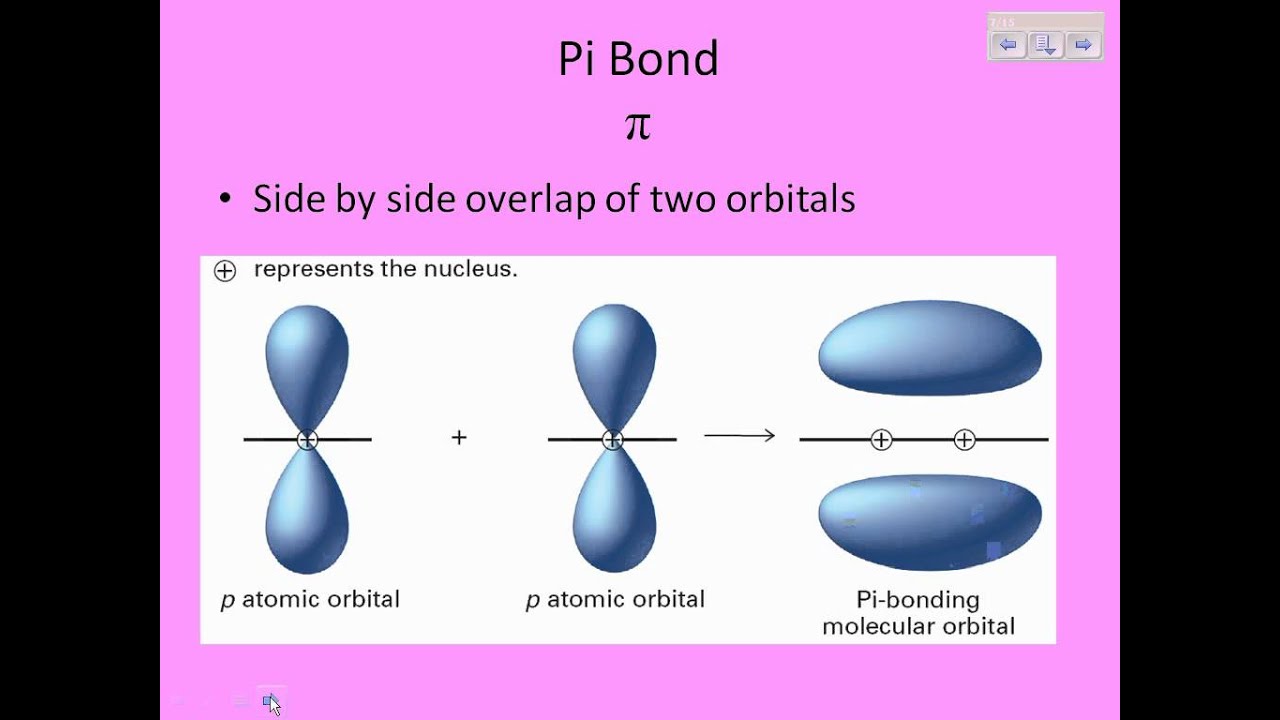
Can someone elaborate upon what it means for a pi bond to have "side-to-side overlap"? Why is this a significant difference to the "end-to-end" overlap of sigma bonds? like, does it give pi bonds any special properties or something along those lines?
Re: "side to side"
Pi bonds are easier to break because they are not as "flexible" as sigma bonds. If the molecules end up rotating, the pi bond will break because it is connected by the side to side overlap.
Re: "side to side"
https://image.shutterstock.com/image-il ... 453236.jpg
Here is a link to a visual representation of a pi bond^
Here is a link to a visual representation of a pi bond^
-
Megan Cai 1H
- Posts: 51
- Joined: Fri Sep 24, 2021 6:53 am
Re: "side to side"
Pi bonds look similar to the number 8 and I think of side to side bonds as the two places of overlap when you have two 8's (88) slightly overlapping each other. These two regions of overlap make it difficult to rotate which is different from sigma bonds, which only have one region of overlap and can rotate easily.
-
Talia Tam 3L
- Posts: 107
- Joined: Fri Sep 24, 2021 5:38 am
Re: "side to side"
The orbitals overlap on either side of the internuclear axis. As somebody else mentioned, they look like two overlapping 8's.
Pi bonds cannot rotate because of this.
Pi bonds cannot rotate because of this.
-
Kristen Bansil 1G
- Posts: 105
- Joined: Fri Sep 24, 2021 7:18 am
Re: "side to side"
What helped me understand the phrase "side to side" as opposed to "end to end" was visualizing two 8's next to each other and instead of overlapping they have a bit of space in between them. Just in case I attached a visual, hope this helped! :)

-
Sarah Wang 1I
- Posts: 106
- Joined: Fri Sep 24, 2021 7:08 am
Re: "side to side"
"side to side" refers to the fact that in pi bonds, the regions of orbital overlap lie on opposite sides of the internuclear axis.
-
Jessica Sun 2I
- Posts: 102
- Joined: Fri Sep 24, 2021 7:18 am
Re: "side to side"
side to side overlapping is important for function of pi bonds, because it means that the bound atoms cannot rotate while sigma bonds do allow for bound atoms to rotate.
-
Janys Li - 1L
- Posts: 52
- Joined: Fri Sep 24, 2021 5:13 am
Re: "side to side"
Hi,
For sigma bonds, the lobes of orbitals align head to tail, meaning that only small regions of electron density overlap, giving the bond great flexibility to rotate around in the molecule. However, for pi bonds, since they are overlapping side to side, large regions of electron density interact with each other, giving them less flexibility to rotate around and causing the atoms to be fixed in place.
For sigma bonds, the lobes of orbitals align head to tail, meaning that only small regions of electron density overlap, giving the bond great flexibility to rotate around in the molecule. However, for pi bonds, since they are overlapping side to side, large regions of electron density interact with each other, giving them less flexibility to rotate around and causing the atoms to be fixed in place.
-
Tony Chen 1F
- Posts: 103
- Joined: Fri Sep 24, 2021 6:59 am
Re: "side to side"
Because sigma bonds are "end to end," a greater region of their electron densities overlap compared to pi bonds.
-
Ananya Sridharan
- Posts: 101
- Joined: Fri Sep 24, 2021 5:46 am
Re: "side to side"
Side to side interactions don't really allow the molecule to rotate or turn like the end to end bonds do. So, pi bonds hold them more tightly and do not allow rotation.
-
Ana Luiza S
- Posts: 29
- Joined: Fri Sep 24, 2021 7:22 am
Re: "side to side"
I think of sigma bonding as axial (along the same axis) overlap of orbitals, while pi bonds are the parallel overlaps of two p orbitals. Kind of like two y axes standing right next to each other.
-
Nomi Heidari-Bateni 2K
- Posts: 103
- Joined: Fri Sep 24, 2021 5:24 am
Re: "side to side"
Sigma bonds are end to end bonds, so there is one region of overlap on the internuclear axis. Since there is only one region of overlap, this bond can rotate. Pi bonds, however, are side to side bonds, so there are two regions of overlap on either side of the internuclear axis. With two regions of overlap, the bond becomes fixed and cannot rotate.
Who is online
Users browsing this forum: No registered users and 5 guests

