strength
Moderators: Chem_Mod, Chem_Admin
-
Roxan Sheikh 3L
- Posts: 114
- Joined: Fri Sep 24, 2021 5:35 am
strength
Generally with sigma and pi bonds, are sigma bonds harder to break than pi? Like which is stronger
-
Maggie Black 1C
- Posts: 101
- Joined: Fri Sep 24, 2021 7:21 am
Re: strength
I think Dr. Lavelle mentioned in one of his lectures that pi bonds are normally easier to break since they are more likely to interact with other molecules, whereas sigma bonds overlap directly and are much closer to the center of the molecule.
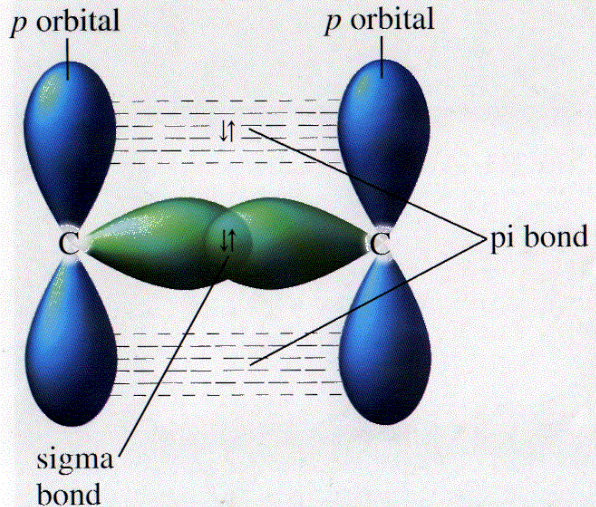

-
Gabrielle Malte 2G
- Posts: 102
- Joined: Fri Sep 24, 2021 6:53 am
Re: strength
A sigma bond is stronger, as it has a larger overlap in orbitals compared to pi bonds. However, when pi bonds are accompanied by sigma bonds, they make the overall bond between 2 atoms stronger
-
Samidha Menon 1E
- Posts: 99
- Joined: Fri Sep 24, 2021 6:39 am
- Been upvoted: 1 time
Re: strength
Sigma bonds, despite being single bonds, are actually stronger because of the overlap in the bonds.
-
Michelle Gong
- Posts: 104
- Joined: Fri Sep 24, 2021 7:10 am
Re: strength
Pi bonds are easier to break because they are formed from electron regions simply being near each other, making them more likely to interact with other molecules. Sigma bonds directly overlap so they are much closer to the center of the molecule and are therefore harder to break.
-
Benicio Rivera 1F
- Posts: 138
- Joined: Fri Sep 24, 2021 6:42 am
Re: strength
Pi bonds are easier to break than sigma bonds. The end-on overlap of orbitals to form a sigma bond is more efficient than the side-on overlap of orbitals to form a pi bond. The electrons in a sigma bond are directly between the two nuclei.
-
Prithvi Raj 3E
- Posts: 106
- Joined: Fri Sep 24, 2021 5:46 am
Re: strength
Sigma bonds are stronger than pi bonds, but because double bonds have both sigma and pi bonds, double bonds are stronger than single bonds.
Re: strength
Pi bonds are weaker and the first to usually break in a reaction. Therefore the sigma bond is stronger.
Re: strength
Pi bonds are weaker and the first to usually break in a reaction. Therefore the sigma bond is stronger.
-
Harbaksh Kaur 3E
- Posts: 50
- Joined: Fri Sep 24, 2021 7:01 am
Re: strength
Sigma bonds are stronger because they overlap directly and are close to the center of an atom.
-
Kelly McFarlane
- Posts: 101
- Joined: Fri Sep 24, 2021 6:02 am
Re: strength
Sigma bonds are stronger than pi bonds because there is only one central region of overlap while pi bonds have two.
Who is online
Users browsing this forum: No registered users and 3 guests

